2'-Deoxy-2'-fluoro-beta-D-arabinonucleosides and oligonucleotides (2'F-ANA): synthesis and physicochemical studies
- PMID: 10982885
- PMCID: PMC110742
- DOI: 10.1093/nar/28.18.3625
2'-Deoxy-2'-fluoro-beta-D-arabinonucleosides and oligonucleotides (2'F-ANA): synthesis and physicochemical studies
Abstract
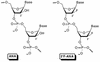
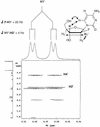
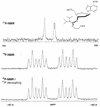
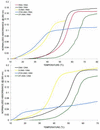
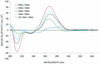
Recently, hybrids of RNA and D-arabinonucleic acids (ANA) as well as the 2'-deoxy-2'-fluoro-D-arabinonucleic acid analog (2'F-ANA) were shown to be substrates of RNase H. This enzyme is believed to be involved in the primary mechanism by which antisense oligonucleotides cause a reduction in target RNA levels in vivo. To gain a better understanding of the properties of arabinose based oligonucleotides, we have prepared a series of 2'F-ANA sequences of homopolymeric (A and T) and mixed base composition (A, T, G and C). UV thermal melting and circular dichroic (CD) studies were used to ascertain the thermodynamic stability and helical conformation of 2'F-ANA/RNA and 2'F-ANA/DNA hybrids. It is shown that 2'F-ANA has enhanced RNA affinity relative to that of DNA and phosphorothioate DNA. The 2'-fluoroarabino modification showed favorable pairing to single-stranded DNA also. This is in sharp contrast to ANA, which forms weak ANA/DNA hybrids at best. According to the measured thermodynamic parameters for duplex formation, the increased stability of hybrids formed by 2'F-ANA (e.g., 2'F-ANA/RNA) appears to originate from conformational pre-organization of the fluorinated sugars and a favorable enthalpy of hybridization. In addition, NMR spectroscopy revealed a five-bond coupling between the 2'F and the base protons (H6/H8) of 2'-deoxy-2'-fluoro-beta-D-arabinonucleosides. This observation is suggestive of a through-space interaction between 2'F and H6/H8 atoms. CD experiments indicate that 2'F-ANA/RNA hybrids adopt an 'A-like' structure and show more resemblance to DNA/RNA hybrids than to the pure RNA/RNA duplex. This feature is believed to be an important factor in the mechanism that allows RNase H to discriminate between 2'F-ANA/RNA (or DNA/RNA) and RNA/RNA duplexes.
Figures

References
-
- Uhlmann E. and Peyman,A. (1990) Chem. Rev., 90, 543–584.
-
- Sanghvi Y. (1989) In Barton,D.H.R., Nakanishi,K. and Meth-Coth,O. (eds), Comprehensive Natural Product Chemistry. Elsevier Science, Oxford, UK, pp. 285–311.
-
- Baker B.F. and Monia,B.P. (1999) Biochem. Biophys. Acta, 1489, 3–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

