Molecular determinants of coordinated proton and zinc inhibition of N-methyl-D-aspartate NR1/NR2A receptors
- PMID: 10984504
- PMCID: PMC27148
- DOI: 10.1073/pnas.180307497
Molecular determinants of coordinated proton and zinc inhibition of N-methyl-D-aspartate NR1/NR2A receptors
Abstract
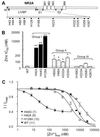
Modulation of the N-methyl-d-aspartate (NMDA)-selective glutamate receptors by extracellular protons and Zn(2+) may play important roles during ischemia in the brain and during seizures. Recombinant NR1/NR2A receptors exhibit a much higher apparent affinity for voltage-independent Zn(2+) inhibition than receptors with other subunit combinations. Here, we show that the mechanism of this apparent high-affinity, voltage-independent Zn(2+) inhibition for NR2A-containing receptors results from the enhancement of proton inhibition. We also show that the N-terminal leucine/isoleucine/valine binding protein (LIVBP)-like domain of the NR2A subunit contains critical determinants of the apparent high-affinity, voltage-independent Zn(2+) inhibition. Mutations H42A, H44G, or H128A greatly increase the Zn(2+) IC(50) (by up to approximately 700-fold) with no effect on the potencies of glutamate and glycine or on voltage-dependent block by Mg(2+). Furthermore, the amino acid residue substitution H128A, which mediates the largest effect on the apparent high-affinity Zn(2+) inhibition among all histidine substitutions we tested, is also critical to the pH-dependency of Zn(2+) inhibition. Our data revealed a unique interaction between two important extracellular modulators of NMDA receptors.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

