Femtosecond dynamics of the forbidden carotenoid S1 state in light-harvesting complexes of purple bacteria observed after two-photon excitation
- PMID: 10984512
- PMCID: PMC27105
- DOI: 10.1073/pnas.190230097
Femtosecond dynamics of the forbidden carotenoid S1 state in light-harvesting complexes of purple bacteria observed after two-photon excitation
Abstract
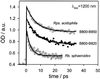
Time-resolved excited-state absorption intensities after direct two-photon excitation of the carotenoid S(1) state are reported for light-harvesting complexes of purple bacteria. Direct excitation of the carotenoid S(1) state enables the measurement of subsequent dynamics on a fs time scale without interference from higher excited states, such as the optically allowed S(2) state or the recently discovered dark state situated between S(1) and S(2). The lifetimes of the carotenoid S(1) states in the B800-B850 complex and B800-B820 complex of Rhodopseudomonas acidophila are 7+/-0.5 ps and 6+/-0.5 ps, respectively, and in the light-harvesting complex 2 of Rhodobacter sphaeroides approximately 1.9+/-0.5 ps. These results explain the differences in the carotenoid to bacteriochlorophyll energy transfer efficiency after S(2) excitation. In Rps. acidophila the carotenoid S(1) to bacteriochlorophyll energy transfer is found to be quite inefficient (phi(ET1) <28%) whereas in Rb. sphaeroides this energy transfer is very efficient (phi(ET1) approximately 80%). The results are rationalized by calculations of the ensemble averaged time constants. We find that the Car S(1) --> B800 electronic energy transfer (EET) pathway ( approximately 85%) dominates over Car S(1) --> B850 EET ( approximately 15%) in Rb. sphaeroides, whereas in Rps. acidophila the Car S(1) --> B850 EET ( approximately 60%) is more efficient than the Car S(1) --> B800 EET ( approximately 40%). The individual electronic couplings for the Car S(1) --> BChl energy transfer are estimated to be approximately 5-26 cm(-1). A major contribution to the difference between the energy transfer efficiencies can be explained by different Car S(1) energy gaps in the two species.
Figures




References
-
- McDermott G, Prince S M, Freer A A, Hawthornthwaitelawless A M, Papiz M Z, Cogdell R J, Isaacs N W. Nature (London) 1995;374:517–521.
-
- McDermott G, Prince S M, Freer A A, Isaacs N W, Papiz M Z, Hawthornthwaitelawless A M, Cogdell R J. Protein Eng. 1995;8:43–43.
-
- Koepke J, Hu X C, Muenke C, Schulten K, Michel H. Structure (London) 1996;4:581–597. - PubMed
-
- Sundstrom V, Pullerits T, van Grondelle R. J Phys Chem B. 1999;103:2327–2346.
-
- Nagarajan V, Johnson E T, Williams J C, Parson W W. J Phys Chem B. 1999;103:2297–2309.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

