A cGMP-signaling pathway in a subset of olfactory sensory neurons
- PMID: 10984544
- PMCID: PMC27070
- DOI: 10.1073/pnas.97.19.10595
A cGMP-signaling pathway in a subset of olfactory sensory neurons
Abstract
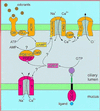
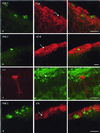
It is well established that signal transduction in sensory neurons of the rat olfactory epithelium involves a cAMP-signaling pathway. However, a small number of olfactory neurons specifically express cGMP-signaling components, namely a guanylyl cyclase (GC-D) and a cGMP-stimulated phosphodiesterase (PDE2). Here, we show that this subset of olfactory neurons expressing GC-D and PDE2 does also express the subunit of a cGMP-selective cyclic nucleotide-gated (CNG) channel that has been previously identified in cone photoreceptors. Further, components of the prototypical cAMP-signaling pathway could not be detected in this subpopulation of cells. These results imply that these neurons use an alternative signaling pathway, with cGMP as the intracellular messenger, and that, in these cells, the receptor current is initiated by the opening of cGMP-gated channels.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

