Isomerization of all-trans-retinol to cis-retinols in bovine retinal pigment epithelial cells: dependence on the specificity of retinoid-binding proteins
- PMID: 10985782
- PMCID: PMC1408314
- DOI: 10.1021/bi001061c
Isomerization of all-trans-retinol to cis-retinols in bovine retinal pigment epithelial cells: dependence on the specificity of retinoid-binding proteins
Abstract
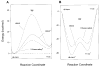
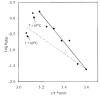
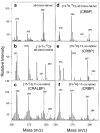
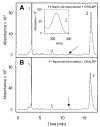
In the retinal rod and cone photoreceptors, light photoactivates rhodopsin or cone visual pigments by converting 11-cis-retinal to all-trans-retinal, the process that ultimately results in phototransduction and visual sensation. The production of 11-cis-retinal in adjacent retinal pigment epithelial (RPE) cells is a fundamental process that allows regeneration of the vertebrate visual system. Here, we present evidence that all-trans-retinol is unstable in the presence of H(+) and rearranges to anhydroretinol through a carbocation intermediate, which can be trapped by alcohols to form retro-retinyl ethers. This ability of all-trans-retinol to form a carbocation could be relevant for isomerization. The calculated activation energy of isomerization of all-trans-retinyl carbocation to the 11-cis-isomer was only approximately 18 kcal/mol, as compared to approximately 36 kcal/mol for all-trans-retinol. This activation energy is similar to approximately 17 kcal/mol obtained experimentally for the isomerization reaction in RPE microsomes. Mass spectrometric (MS) analysis of isotopically labeled retinoids showed that isomerization proceeds via alkyl cleavage mechanism, but the product of isomerization depended on the specificity of the retinoid-binding protein(s) as evidenced by the production of 13-cis-retinol in the presence of cellular retinoid-binding protein (CRBP). To test the influence of an electron-withdrawing group on the polyene chain, which would inhibit carbocation formation, 11-fluoro-all-trans-retinol was used in the isomerization assay and was shown to be inactive. Together, these results strengthen the idea that the isomerization reaction is driven by mass action and may occur via carbocation intermediate.
Figures





References
-
- Palczewski K, Polans A, Baehr W, Ames JB. BioEssays. 2000;22:337–350. - PubMed
-
- Polans A, Baehr W, Palczewski K. Trends Neurosci. 1996;19:547–554. - PubMed
-
- Lagndao L, Baylor D. Neuron. 1992;8:995–1002. - PubMed
-
- Koutalos Y, Yau KW. Trends Neurosci. 1996;19:73–81. - PubMed
-
- Pugh EN, Jr, Nikonov S, Lamb TD. Curr Opin Neurobiol. 1999;9:410–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

