Short course acid suppressive treatment for patients with functional dyspepsia: results depend on Helicobacter pylori status. The Frosch Study Group
- PMID: 10986206
- PMCID: PMC1728071
- DOI: 10.1136/gut.47.4.473
Short course acid suppressive treatment for patients with functional dyspepsia: results depend on Helicobacter pylori status. The Frosch Study Group
Abstract
Background and aims: Treatment of functional dyspepsia with acid inhibitors is controversial and it is not known if the presence of Helicobacter pylori infection influences the response.
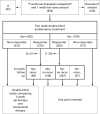
Methods: After a complete diagnostic workup, 792 patients with functional dyspepsia unresponsive to one week of low dose antacid treatment were randomised to two weeks of treatment with placebo, ranitidine 150 mg, omeprazole 10 mg, or omeprazole 20 mg daily. Individual dyspeptic and other abdominal symptoms were evaluated before and after treatment according to H pylori status.
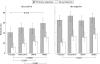
Results: The proportions of patients considered to be in remission (intention to treat) at the end of treatment with placebo, ranitidine 150 mg, omeprazole 10 mg, and omeprazole 20 mg were, respectively, 42%, 50%, 48%, and 59% in the H pylori positive group and 66%, 73%, 64%, and 71% in the H pylori negative group. In H pylori positive patients, the therapeutic gain over placebo was significant for omeprazole 20 mg (17.6%, 95% confidence intervals (CI) 4.2-31.0; p<0.014 using the Bonferroni-adjusted p level of 0.017) but not for omeprazole 10 mg (6.8%, 95% CI -6.7-20.4) or ranitidine 150 mg (8.9%, 95% CI -4.2-21. 9). There was no significant therapeutic gain from active treatment over placebo in H pylori negative patients. Complete disappearance of symptoms and improvement in quality of life also occurred most frequently with omeprazole 20 mg and was significant in both H pylori positive and H pylori negative groups. The six month relapse rate of symptoms requiring treatment was low (<20%) in all groups.
Conclusions: Omeprazole 20 mg per day had a small but significant favourable effect on outcome in H pylori positive patients. The differential response in these patients may be explained by an enhanced antisecretory response in the presence of H pylori. The effect of weaker acid inhibition was unsatisfactory.
Figures


Comment in
-
No H pylori: less dyspepsia?Gut. 2000 Oct;47(4):461-2. doi: 10.1136/gut.47.4.461. Gut. 2000. PMID: 10986201 Free PMC article. No abstract available.
-
H pylori and functional dyspepsia.Gut. 2001 Nov;49(5):738-9. doi: 10.1136/gut.49.5.738a. Gut. 2001. PMID: 11693119 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
