Characterization of three XylT-like [2Fe-2S] ferredoxins associated with catabolism of cresols or naphthalene: evidence for their involvement in catechol dioxygenase reactivation
- PMID: 10986264
- PMCID: PMC111004
- DOI: 10.1128/JB.182.19.5580-5585.2000
Characterization of three XylT-like [2Fe-2S] ferredoxins associated with catabolism of cresols or naphthalene: evidence for their involvement in catechol dioxygenase reactivation
Abstract
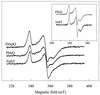
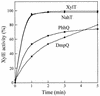
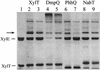
The xylT gene product, a component of the xylene catabolic pathway of Pseudomonas putida mt2, has been recently characterized as a novel [2Fe-2S] ferredoxin which specifically reactivates oxygen-inactivated catechol 2,3-dioxygenase (XylE). In this study, three XylT-like proteins potentially involved in the catabolism of naphthalene (NahT) or cresols (PhhQ and DmpQ) have been overexpressed in Escherichia coli, purified, and compared with respect to their biochemical properties and interaction with XylE. The three XylT analogues show general spectroscopic characteristics common to plant-type [2Fe-2S] ferredoxins as well as distinctive features that appear to be typical for the XylT subgroup of these proteins. The midpoint redox potentials of the PhhQ and DmpQ proteins were -286 mV and -323 mV, respectively. Interestingly, all purified XylT-like proteins promoted in vitro reactivation of XylE almost as efficiently as XylT. The interaction of XylE with XylT and its analogues was studied by cross-linking experiments using the 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. A polypeptide band with an M(r) of 46,000, which corresponded to the cross-linked product between one XylE subunit and one molecule of ferredoxin, was obtained in all cases. The formation of the complex was affected by ionic strength, indicating that electrostatic forces are involved in the dioxygenase-ferredoxin interaction. In complementation experiments, plasmids expressing xylT or its analogues were introduced into an XylT-null mutant of P. putida which is unable to grow on p-methylbenzoate. All transconjugants regained the wild-type phenotype, indicating that all analogues can substitute for XylT in the in vivo reactivation of XylE. Our results provide evidence for a subgroup of [2Fe-2S] ferredoxins with distinct biochemical properties whose specific function is to reactivate intrinsically labile extradiol ring cleavage dioxygenases involved in the catabolism of various aromatic hydrocarbons.
Figures

References
-
- Bosch R, Garcia-Valdes E, Moore E R. Complete nucleotide sequence and evolutionary significance of a chromosomally encoded naphthalene-degradation lower pathway from Pseudomonas stutzeri AN10. Gene. 2000;245:65–74. - PubMed
-
- Brandt M E, Vickery L E. Charge pair interactions stabilizing ferredoxin-ferredoxin reductase complexes. Identification by complementary site-specific mutations. J Biol Chem. 1993;268:17126–17130. - PubMed
-
- Coghlan V M, Vickery L E. Site-specific mutations in human ferredoxin that affect binding to ferredoxin reductase and cytochrome P450scc. J Biol Chem. 1991;266:18606–18612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

