Activation of the beta globin locus by transcription factors and chromatin modifiers
- PMID: 10990462
- PMCID: PMC314215
- DOI: 10.1093/emboj/19.18.4986
Activation of the beta globin locus by transcription factors and chromatin modifiers
Abstract
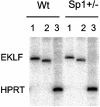
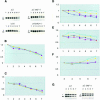
Locus control regions (LCRs) alleviate chromatin-mediated transcriptional repression. Incomplete LCRs partially lose this property when integrated in transcriptionally restrictive genomic regions such as centromeres. This frequently results in position effect variegation (PEV), i.e. the suppression of expression in a proportion of the cells. Here we show that this PEV is influenced by the heterochromatic protein SUV39H1 and by the Polycomb group proteins M33 and BMI-1. A concentration variation of these proteins modulates the proportion of cells expressing human globins in a locus-dependent manner. Similarly, the transcription factors Sp1 or erythroid Krüppel-like factor (EKLF) also influence PEV, characterized by a change in the number of expressing cells and the chromatin structure of the locus. However, in contrast to results obtained in a euchromatic locus, EKLF influences the expression of the gamma- more than the beta-globin genes, suggesting that the relief of silencing is caused by the binding of EKLF to the LCR and that genes at an LCR proximal position are more likely to be in an open chromatin state than genes at a distal position.
Figures



Similar articles
-
The role of EKLF in human beta-globin gene competition.Genes Dev. 1996 Nov 15;10(22):2894-902. doi: 10.1101/gad.10.22.2894. Genes Dev. 1996. PMID: 8918890
-
The binding of the ubiquitous transcription factor Sp1 at the locus control region represses the expression of beta-like globin genes.Proc Natl Acad Sci U S A. 2005 Jul 12;102(28):9896-900. doi: 10.1073/pnas.0502041102. Epub 2005 Jul 5. Proc Natl Acad Sci U S A. 2005. PMID: 15998736 Free PMC article.
-
Erythroid Krüppel-like factor (EKLF) is active in primitive and definitive erythroid cells and is required for the function of 5'HS3 of the beta-globin locus control region.EMBO J. 1998 Apr 15;17(8):2334-41. doi: 10.1093/emboj/17.8.2334. EMBO J. 1998. PMID: 9545245 Free PMC article.
-
Transcriptional factors for specific globin genes.Ann N Y Acad Sci. 1998 Jun 30;850:64-9. doi: 10.1111/j.1749-6632.1998.tb10463.x. Ann N Y Acad Sci. 1998. PMID: 9668528 Review.
-
Developmental control of epsilon- and gamma-globin genes.Ann N Y Acad Sci. 1998 Jun 30;850:10-7. doi: 10.1111/j.1749-6632.1998.tb10457.x. Ann N Y Acad Sci. 1998. PMID: 9668523 Review.
Cited by
-
Distinct domains of erythroid Krüppel-like factor modulate chromatin remodeling and transactivation at the endogenous beta-globin gene promoter.Mol Cell Biol. 2002 Jan;22(1):161-70. doi: 10.1128/MCB.22.1.161-170.2002. Mol Cell Biol. 2002. PMID: 11739731 Free PMC article.
-
The epigenetic stability of the locus control region-deficient IgH locus in mouse hybridoma cells is a clonally varying, heritable feature.Genetics. 2004 May;167(1):411-21. doi: 10.1534/genetics.167.1.411. Genetics. 2004. PMID: 15166165 Free PMC article.
-
Embryonic but not postnatal reexpression of hepatocyte nuclear factor 1alpha (HNF1alpha) can reactivate the silent phenylalanine hydroxylase gene in HNF1alpha-deficient hepatocytes.Mol Cell Biol. 2001 Jun;21(11):3662-70. doi: 10.1128/MCB.21.11.3662-3670.2001. Mol Cell Biol. 2001. PMID: 11340160 Free PMC article.
-
Alterations in expression and chromatin configuration of the alpha hemoglobin-stabilizing protein gene in erythroid Kruppel-like factor-deficient mice.Mol Cell Biol. 2006 Jun;26(11):4368-77. doi: 10.1128/MCB.02216-05. Mol Cell Biol. 2006. PMID: 16705186 Free PMC article.
-
The human beta-globin locus control region can silence as well as activate gene expression.Mol Cell Biol. 2005 May;25(10):3864-74. doi: 10.1128/MCB.25.10.3864-3874.2005. Mol Cell Biol. 2005. PMID: 15870261 Free PMC article.
References
-
- Aagaard L., Schmid,M., Warburton,P. and Jenuwein,T. (2000) Mitotic phosphorylation of SUV39H1, a novel component of active centromeres, coincides with transient accumulation at mammalian centromeres. J. Cell Sci., 113, 817–829. - PubMed
-
- Alami R., Greally,J.M., Tanimoto,K., Hwang,S., Feng,Y.Q., Engel,J.D., Fiering,S. and Bouhassira,E.E. (2000) β-globin YAC transgenes exhibit uniform expression levels but position effect variegation in mice. Hum. Mol. Genet., 9, 631–636. - PubMed
-
- Alkema M.J., van der Lugt,N.M.T., Bobeldijk,R.C., Berns,A. and van Lohuizen,M. (1995) Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature, 374, 724–727. - PubMed
-
- Aparicio O.M. and Gottschling,D.E. (1994) Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev., 8, 1133–1146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

