Structural elements near the C-terminus are responsible for changes in nicotinic receptor gating kinetics following patch excision
- PMID: 10990529
- PMCID: PMC2270086
- DOI: 10.1111/j.1469-7793.2000.t01-2-00405.x
Structural elements near the C-terminus are responsible for changes in nicotinic receptor gating kinetics following patch excision
Abstract
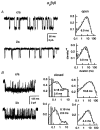
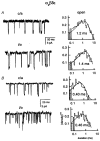
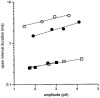
We have studied the effect of patch excision on the gating kinetics of muscle nicotinic acetylcholine receptors transiently expressed in HEK 293 cells. The experiments were performed on embryonic and adult wild-type, and several mutated, receptors using acetylcholine, carbamylcholine and tetramethylammonium as agonists. We show that patch excision of cell-attached patches into the inside-out configuration led to a reduction of mean open duration in receptors containing a gamma-subunit (embryonic) but not an epsilon-subunit (adult receptors). Kinetic analysis of an embryonic receptor containing a mutated residue, alphaY93F, showed that the reduction in the mean open duration upon patch excision was mainly caused by an increase in the channel closing rate constant. This was confirmed by experiments on embryonic wild-type receptors using carbamylcholine as an agonist with low efficacy. By expressing receptors containing chimeric gamma-epsilon subunits we found that segments of the gamma-subunit corresponding to a region within the M3-M4 linker (the amphipathic helix, HA) and the M4 transmembrane domain were required for the reduction in channel open duration after excision. The results indicate that particular residues in both M4 and HA are required to allow the change in open time after excision. This finding suggests that there is an interaction between these two regions in determining the modulation of gating kinetics.
Figures
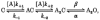
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

