Regulation of voltage-dependent calcium channels in rat sensory neurones involves a Ras-mitogen-activated protein kinase pathway
- PMID: 10990531
- PMCID: PMC2270090
- DOI: 10.1111/j.1469-7793.2000.00433.x
Regulation of voltage-dependent calcium channels in rat sensory neurones involves a Ras-mitogen-activated protein kinase pathway
Abstract
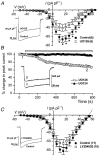
The small G-protein Ras, a critical component in the signalling pathways regulating cell growth, is involved in the tonic upregulation of voltage-dependent calcium channels (VDCCs) in rat sensory neurones. To investigate which downstream effector(s) of Ras is involved in this process, a series of Ras mutant cDNAs were co-expressed with green fluorescent protein (GFP) in primary cultured rat dorsal root ganglion neurones (DRGs). Constitutively active V12Ras (glycine 12 to valine) markedly increased basal calcium current density by 41 % compared with control cells (GFP alone). In contrast, a farnesylation-defective mutant, V12S186Ras (cysteine 186 to serine; activates no downstream effectors), significantly reduced calcium current density by 47 %. Ras effector region mutants V12C40 (tyrosine 40 to cysteine; activates the p110 alpha-subunit of phosphatidylinositol 3-kinase) and V12G37 (glutamic acid 37 to glycine; activates Ral guanine nucleotide dissociation stimulator) had no significant effect on VDCC current. However, V12S35Ras (threonine 35 to serine; activates Raf-1 and the mitogen-activated protein kinase (MAPK) pathway) markedly increased basal calcium current density by 67 %, suggesting that Raf-1 activation is sufficient for Ras enhancement of calcium current in these cells. Raf-1 activates MEK (MAPK kinase) in the MAPK pathway, and the MEK inhibitor U0126 reduced calcium current by 45 % after 10-15 min, whereas the inactive analogue U0124 had no effect. This rapid time course for MEK inhibition suggests direct modulation of VDCCs via the Ras-MAPK pathway rather than gene expression-mediated effects. The relative proportions of omega-conotoxin GVIA- and nicardipine-sensitive N- ( approximately 40 %) and L- ( approximately 40 %) type currents were unaffected by either V12S35Ras expression or U0126 pre-treatment, suggesting that all components of calcium current in DRGs, are enhanced via this pathway.
Figures




Similar articles
-
The presence of Ca2+ channel beta subunit is required for mitogen-activated protein kinase (MAPK)-dependent modulation of alpha1B Ca2+ channels in COS-7 cells.J Physiol. 2002 Sep 1;543(Pt 2):425-37. doi: 10.1113/jphysiol.2002.022822. J Physiol. 2002. PMID: 12205179 Free PMC article.
-
Regulation of rat neuronal voltage-dependent calcium channels by endogenous p21-ras.Eur J Neurosci. 1997 Jun;9(6):1252-61. doi: 10.1111/j.1460-9568.1997.tb01480.x. Eur J Neurosci. 1997. PMID: 9215709
-
Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors.Mol Cell Neurosci. 2003 Jan;22(1):118-32. doi: 10.1016/s1044-7431(02)00022-2. Mol Cell Neurosci. 2003. PMID: 12595244
-
Structural insights into the role of SHOC2-MRAS-PP1C complex in RAF activation.FEBS J. 2023 Oct;290(20):4852-4863. doi: 10.1111/febs.16800. Epub 2023 Apr 26. FEBS J. 2023. PMID: 37074066 Free PMC article. Review.
-
Mosaic RASopathies: A review of disorders caused by somatic pathogenic variants in the genes of the RAS/MAPK pathway.Am J Med Genet C Semin Med Genet. 2022 Dec;190(4):520-529. doi: 10.1002/ajmg.c.32021. Epub 2022 Dec 2. Am J Med Genet C Semin Med Genet. 2022. PMID: 36461154 Review.
Cited by
-
The Na/K-ATPase Signaling: From Specific Ligands to General Reactive Oxygen Species.Int J Mol Sci. 2018 Sep 1;19(9):2600. doi: 10.3390/ijms19092600. Int J Mol Sci. 2018. PMID: 30200500 Free PMC article. Review.
-
Beta subunits of voltage-gated calcium channels.J Bioenerg Biomembr. 2003 Dec;35(6):599-620. doi: 10.1023/b:jobb.0000008026.37790.5a. J Bioenerg Biomembr. 2003. PMID: 15000522 Review.
-
Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization.J Neurosci. 2002 Sep 1;22(17):7737-45. doi: 10.1523/JNEUROSCI.22-17-07737.2002. J Neurosci. 2002. PMID: 12196597 Free PMC article.
-
mTORC1 inhibition induces pain via IRS-1-dependent feedback activation of ERK.Pain. 2013 Jul;154(7):1080-91. doi: 10.1016/j.pain.2013.03.021. Epub 2013 Mar 15. Pain. 2013. PMID: 23607966 Free PMC article.
-
Prokineticin 2 depolarizes paraventricular nucleus magnocellular and parvocellular neurons.Eur J Neurosci. 2007 Jan;25(2):425-34. doi: 10.1111/j.1460-9568.2006.05293.x. Eur J Neurosci. 2007. PMID: 17284183 Free PMC article.
References
-
- DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase kinase blocks T-cell proliferation but does not induce or prevent anergy. Journal of Immunology. 1998;160:4175–4181. - PubMed
-
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos PA. Identification of a novel inhibitor of mitogen activated protein kinase kinase. Journal of Biological Chemistry. 1998;273:18623–18632. - PubMed
-
- Finkbeiner S, Greenberg ME. Ca2+-dependent routes to Ras: mechanisms for neuronal survival, differentiation, and plasticity? Neuron. 1996;16:233–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

