Modulation of slow inactivation in class A Ca2+ channels by beta-subunits
- PMID: 10990532
- PMCID: PMC2270100
- DOI: 10.1111/j.1469-7793.2000.t01-1-00445.x
Modulation of slow inactivation in class A Ca2+ channels by beta-subunits
Abstract
beta-subunit modulation of slow inactivation of class A calcium (Ca2+) channels was studied with two-microlectrode voltage clamp after expression of the alpha1A- (BI-2) together with beta1a-, beta2a-, beta3- or beta4-subunits in Xenopus oocytes. On- and off-rates of slow inactivation were estimated from the kinetics of recovery from slow inactivation. Ca2+ channels with an alpha1A/beta-subunit composition inducing the slower rate of fast inactivation displayed the faster rate of slow inactivation. The corresponding order of slow inactivation time constants (tau[onset]) was: alpha1A/beta2a, 33 +/- 3 s; alpha1A/beta4, 42 +/- 4 s; alpha1A/beta1a, 59 +/- 4 s; alpha1A/beta3, 67 +/- 5 s (n >= 7). Recovery of class A Ca2+ channels from slow inactivation was voltage dependent and accelerated at hyperpolarized voltages. At a given holding potential recovery kinetics were not significantly modulated by different beta-subunits. Two mutations in segment IIIS6 (IF1612/1613AA) slowed fast inactivation and accelerated the onset of slow inactivation in the resulting mutant (alpha1A/IF-AA/beta3) in a similar manner as coexpression of the beta2a-subunit. Recovery from slow inactivation was slightly slowed in the double mutant. Our data suggest that class A Ca2+ channels enter the 'slow inactivated' state more willingly from the open than from the 'fast inactivated' state. The rate of slow inactivation is, therefore, indirectly modulated by different beta-subunits. Fast and slow inactivation in class A Ca2+ channels appears to represent structurally independent conformational changes. Fast inactivation is not a prerequisite for slow inactivation.
Figures

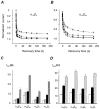
 ) −80 mV (▪) and −60 mV (▪). D, time constants of recovery from slow inactivation at −120 mV (□), −80 mV (▪) and −60 mV (
) −80 mV (▪) and −60 mV (▪). D, time constants of recovery from slow inactivation at −120 mV (□), −80 mV (▪) and −60 mV ( ) were estimated as described in Fig. 2A (n > 20). No significant differences with τslow estimated from double pulse experiments (Table 1) were observed (P > 0.05). The mean values for different subunit combinations at the corresponding holding potentials were not significantly different (P > 0.05).
) were estimated as described in Fig. 2A (n > 20). No significant differences with τslow estimated from double pulse experiments (Table 1) were observed (P > 0.05). The mean values for different subunit combinations at the corresponding holding potentials were not significantly different (P > 0.05).


References
-
- Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. - PubMed
-
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nature Neuroscience. 1999;5:407–415. - PubMed
-
- Boyett MR, Honjo H, Harrison SM, Zang W-J, Kirby MS. Ultra-slow voltage-dependent inactivation of the calcium current in guinea-pig and ferret ventricular myocytes. Pflügers Archiv. 1994;428:39–50. - PubMed
-
- Burgess DL, Biddlecome GH, McDonough SI, Diaz ME, Zilinski CA, Bean BP, Campbell KP, Noebels JL. β-subunit reshuffling modifies N- and P/Q-type Ca2+ channel subunit compositions in lethargic mouse brain. Molecular and Cellular Neuroscience. 1999;13:293–311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

