Molecular evidence for a role of Shaw (Kv3) potassium channel subunits in potassium currents of dog atrium
- PMID: 10990534
- PMCID: PMC2270093
- DOI: 10.1111/j.1469-7793.2000.00467.x
Molecular evidence for a role of Shaw (Kv3) potassium channel subunits in potassium currents of dog atrium
Abstract
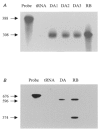
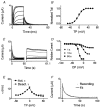
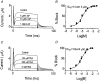
We previously described an ultrarapid delayed rectifier current in dog atrial myocytes (IKur,d) with properties resembling currents reported for Kv3.1 channels in neural tissue; however, there was no direct molecular evidence for Shaw subfamily (Kv3) subunit expression in the heart. To identify the molecular basis of IKur,d, we cloned a full-length cDNA (dKv3.1) from canine atrium with homology-based reverse transcription (RT)- polymerase chain reaction (PCR) cloning techniques. A 1755 bp full-length cDNA (dKv3.1) was obtained, with 94.2 % homology to rat brain Kv3.1 (rbKv3.1). The deduced amino acid sequence had 99.3 % homology with rbKv3.1. Heterologous expression of dKv3.1 in Xenopus oocytes produced currents with activation voltage dependence, rectification, and activation and deactivation kinetics that strongly resemble native IKur,d. Like IKur,d, dKv3.1 was found to be highly sensitive to extracellular 4-aminopyridine (4-AP) and tetraethylammonium (TEA). RNase protection assays, Western blots and immunohistochemical studies demonstrated the presence of dKv3.1 transcripts and proteins in dog atrial preparations and isolated canine atrial myocytes. Protein corresponding to the Kv1.5 subunit, which can also carry ultrarapid delayed rectifier current, was absent. Unlike neural tissues, which express two splice variants (Kv3.1a and Kv3.1b), canine atrium showed only Kv3.1b transcripts. Whole-cell patch-clamp studies showed that IKur,d is absent in canine ventricular myocytes, and immunohistochemical and Western blot analysis demonstrated the absence of dKv3.1 protein in canine ventricle. We conclude that the Shaw-type channel dKv3.1 is present in dog atrium, but not ventricle, and is the likely molecular basis of canine atrial IKur,d.
Figures





Similar articles
-
Characterization of an ultrarapid delayed rectifier potassium channel involved in canine atrial repolarization.J Physiol. 1996 Nov 1;496 ( Pt 3)(Pt 3):647-62. doi: 10.1113/jphysiol.1996.sp021716. J Physiol. 1996. PMID: 8930833 Free PMC article.
-
Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes.Circ Res. 2003 Oct 17;93(8):744-51. doi: 10.1161/01.RES.0000096362.60730.AE. Epub 2003 Sep 18. Circ Res. 2003. PMID: 14500335
-
Expression of voltage-dependent K(+) channel genes in mesenteric artery smooth muscle cells.Am J Physiol. 1999 Nov;277(5):G1055-63. doi: 10.1152/ajpgi.1999.277.5.G1055. Am J Physiol. 1999. PMID: 10564112
-
When, where, and how much? Expression of the Kv3.1 potassium channel in high-frequency firing neurons.J Neurobiol. 1998 Oct;37(1):69-79. doi: 10.1002/(sici)1097-4695(199810)37:1<69::aid-neu6>3.0.co;2-6. J Neurobiol. 1998. PMID: 9777733 Review.
-
Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing.Trends Neurosci. 2001 Sep;24(9):517-26. doi: 10.1016/s0166-2236(00)01892-0. Trends Neurosci. 2001. PMID: 11506885 Review.
Cited by
-
MinK-related peptide 2 modulates Kv2.1 and Kv3.1 potassium channels in mammalian brain.J Neurosci. 2003 Sep 3;23(22):8077-91. doi: 10.1523/JNEUROSCI.23-22-08077.2003. J Neurosci. 2003. PMID: 12954870 Free PMC article.
-
Rate-Dependent Role of IKur in Human Atrial Repolarization and Atrial Fibrillation Maintenance.Biophys J. 2017 May 9;112(9):1997-2010. doi: 10.1016/j.bpj.2017.03.022. Biophys J. 2017. PMID: 28494969 Free PMC article.
-
Ion Channels in the Heart.Compr Physiol. 2015 Jul 1;5(3):1423-64. doi: 10.1002/cphy.c140069. Compr Physiol. 2015. PMID: 26140724 Free PMC article. Review.
-
Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties.J Physiol. 2003 Sep 15;551(Pt 3):801-13. doi: 10.1113/jphysiol.2003.046417. Epub 2003 Jul 7. J Physiol. 2003. PMID: 12847206 Free PMC article.
-
Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current.J Physiol. 2003 May 15;549(Pt 1):65-74. doi: 10.1113/jphysiol.2003.039859. Epub 2003 Apr 4. J Physiol. 2003. PMID: 12679374 Free PMC article.
References
-
- Abbott GW, Sesti F, Spawski I, Buck M E, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms Ikr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- Barry DM, Nerbonne JM. Myocardial potassium channels: electrophysiological and molecular diversity. Annual Review of Physiology. 1996;58:363–394. - PubMed
-
- Brahmajothi MV, Morales MJ, Liu S, Rasmusson RL, Campbell DL, Strauss HC. In situ hybridization reveals extensive diversity of K+ channel mRNA in isolated ferret cardiac myocytes. Circulation Research. 1996;78:1083–1089. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

