Inhibition of a mammalian large conductance, calcium-sensitive K+ channel by calmodulin-binding peptides
- PMID: 10990535
- PMCID: PMC2270083
- DOI: 10.1111/j.1469-7793.2000.00479.x
Inhibition of a mammalian large conductance, calcium-sensitive K+ channel by calmodulin-binding peptides
Abstract
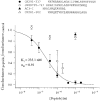
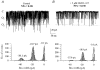
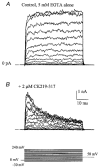
The large conductance, calcium-sensitive K+ channel (BKCa channel) is a voltage-activated ion channel in which direct calcium binding shifts gating to more negative cellular membrane potentials. We hypothesized that the calcium-binding domain of BKCa channels may mimic the role played by calmodulin (CaM) in the activation of calcium-CaM-dependent enzymes, in which a tonic inhibitory constraint is removed on CaM binding. To examine such a hypothesis, we used peptides from the autoregulatory domains of CaM kinase II (CK291-317) and cNOS (the constitutive nitric oxide synthase; cNOS725-747) as probes for the calcium-dependent activation of murine BKCa channels transiently expressed in HEK 293 cells. We found that these CaM-binding peptides produced potent, time-dependent inhibition of mammalian BKCa channel current following voltage-dependent activation. Inhibition was observed in both the presence and the absence of cytosolic free calcium. Similar application of CK291-31 had no effect on either the amplitude or kinetics of voltage-dependent, macroscopic currents recorded from rabbit smooth muscle Kv1.5 potassium channels transiently expressed in HEK 293 cells. Cytosolic application of both CK291-317 and tetraethylammonium (TEA) produced an additive and non-competitive block of BKCa current. This finding suggests that the peptide-binding site is distinct (e.g. outside the pore region of the channel) from that of TEA. Our results are thus consistent with a model in which the BKCa channel's voltage-dependent gating process is under an intramolecular constraint that is relieved upon calcium binding. The intrinsic calcium sensor of the channel may thus interact with an inhibitory domain present in the BKCa channel, and by doing so, remove an inhibitory 'constraint' that permits voltage-dependent gating to occur at more negative potentials.
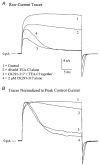
Figures





Similar articles
-
Block of large conductance Ca(2+)-activated K+ channels in rabbit vascular myocytes by internal Mg2+ and Na+.J Physiol. 1996 Sep 15;495 ( Pt 3)(Pt 3):701-16. doi: 10.1113/jphysiol.1996.sp021627. J Physiol. 1996. PMID: 8887777 Free PMC article.
-
Ammonium ion enhances the calcium-dependent gating of a mammalian large conductance, calcium-sensitive K+ channel.Can J Physiol Pharmacol. 2001 Nov;79(11):919-23. Can J Physiol Pharmacol. 2001. PMID: 11760093
-
Molecular and functional identification of cyclic AMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells.Circ Res. 2003 Aug 8;93(3):213-20. doi: 10.1161/01.RES.0000085041.70276.3D. Epub 2003 Jul 3. Circ Res. 2003. PMID: 12842920
-
Small proteins that modulate calmodulin-dependent signal transduction: effects of PEP-19, neuromodulin, and neurogranin on enzyme activation and cellular homeostasis.Mol Neurobiol. 2000 Aug-Dec;22(1-3):99-113. doi: 10.1385/MN:22:1-3:099. Mol Neurobiol. 2000. PMID: 11414283 Review.
-
Small conductance calcium-activated potassium channels: from structure to function.Prog Neurobiol. 2010 Jul;91(3):242-55. doi: 10.1016/j.pneurobio.2010.03.002. Epub 2010 Mar 30. Prog Neurobiol. 2010. PMID: 20359520 Review.
Cited by
-
Calmodulin kinase II inhibition disrupts cardiomyopathic effects of enhanced green fluorescent protein.J Mol Cell Cardiol. 2008 Feb;44(2):405-10. doi: 10.1016/j.yjmcc.2007.10.014. Epub 2007 Nov 28. J Mol Cell Cardiol. 2008. PMID: 18048055 Free PMC article.
-
A protein interaction network for the large conductance Ca(2+)-activated K(+) channel in the mouse cochlea.Mol Cell Proteomics. 2009 Aug;8(8):1972-87. doi: 10.1074/mcp.M800495-MCP200. Epub 2009 May 7. Mol Cell Proteomics. 2009. PMID: 19423573 Free PMC article.
-
Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons.J Neurophysiol. 2011 Apr;105(4):1651-9. doi: 10.1152/jn.00058.2011. Epub 2011 Feb 9. J Neurophysiol. 2011. PMID: 21307321 Free PMC article.
-
Contribution of potential EF hand motifs to the calcium-dependent gating of a mouse brain large conductance, calcium-sensitive K(+) channel.J Physiol. 2001 Jun 15;533(Pt 3):681-95. doi: 10.1111/j.1469-7793.2001.00681.x. J Physiol. 2001. PMID: 11410626 Free PMC article.
References
-
- Asano M, Nomura Y, Ito K, Uyama Y, Imaizumi Y, Watanabe M. Increased function of voltage-dependent Ca2+ channels and Ca2+-activated K+ channels in resting state of femoral arteries from spontaneously hypertensive rats at prehypertensive stage. Journal of Pharmacology and Experimental Therapeutics. 1995;275:775–783. - PubMed
-
- Blatz AL, Magleby KL. Calcium-activated potassium channels. Trends in Neurosciences. 1987;10:463–467.
-
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annual Review of Physiology. 1995b;57:417–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

