Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons
- PMID: 10990538
- PMCID: PMC2270092
- DOI: 10.1111/j.1469-7793.2000.00515.x
Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons
Abstract
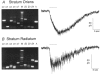
The molecular and functional properties of neuronal nicotinic acetylcholine receptors (nAChRs) were characterized from CA1 neurons in rat hippocampal slices using single-cell reverse-transcription polymerase chain reaction (RT-PCR) in conjunction with whole-cell patch-clamp recordings. We analysed the presence of the neuronal nAChR subunit mRNAs alpha2-7 and beta2-4, along with the mRNA for the GABAergic markers GAD (glutamic acid decarboxylase) 65 and 67 isoforms, and VGAT (vesicular GABA transporter) in interneurons from the stratum radiatum and stratum oriens, and in CA1 pyramidal neurons. Functional nAChR-mediated currents were detected in both interneuron populations, but not in pyramidal neurons. The neuronal nAChR subunit mRNAs detected in > 20 % of the populations examined were alpha4, alpha5, alpha7 and beta2-4 in stratum radiatum interneurons; alpha2, alpha3, alpha4, alpha7, beta2 and beta3 subunits in stratum oriens interneurons; and beta2-4 in pyramidal neurons. High levels of GABAergic marker mRNAs were detected in both interneuron populations, but not in pyramidal neurons. Significant co-expression of nAChR subunits within individual neurons was detected for alpha3 + alpha5, alpha4 + beta2, alpha4 + beta3, alpha7 + beta2, beta2 + beta3 and beta3 + beta4. The kinetics of the nAChR-mediated currents in response to the application of 100 microM ACh were significantly correlated with the expression of particular nAChR subunits. The alpha3, alpha7 and beta2 nAChR subunits were individually correlated with a fast rise time, the alpha2 nAChR subunit was correlated with a medium rise time, and the alpha4 nAChR subunit was correlated with a slow rise time. The alpha2 and alpha4 nAChR subunits were also significantly correlated with slow desensitization kinetics.
Figures

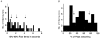
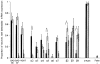
References
-
- Albuquerque EX, Pereira EFR, Castro NG, Alkondon M, Reinhardt S, Schroder H, Maelicke A. Nicotinic receptor function in the mammalian central nervous system. Annals of the New York Academy of Sciences. 1995;757:48–72. - PubMed
-
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. - PubMed
-
- Alkondon M, Pereira EFR, Albuquerque EX. α-Bungarotoxin- and methyllcaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Research. 1998;810:257–263. - PubMed
-
- Alkondon M, Pereira EFR, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates γ-aminobutyric acid release from CA1 neurons of rat hippocampal slices. Journal of Pharmacology and Experimental Therapeutics. 1997;283:1396–1411. - PubMed
-
- Anand R, Peng X, Lindstrom J. Homomeric and native α7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Letters. 1993;327:241–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

