Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography
- PMID: 10990546
- PMCID: PMC2270094
- DOI: 10.1111/j.1469-7793.2000.00623.x
Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography
Abstract
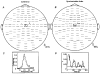
Magnetoencephalographic (MEG) and electromyographic (EMG) signals were recorded from six subjects during isometric contraction of four different muscles. Cortical sources were located from the MEG signal which was averaged time-locked to the onset of motor unit potentials. A spatial filtering algorithm was used to estimate the source activity. Sources were found in the primary motor cortex (M1) contralateral to the contracted muscle. Significant coherence between rectified EMG and M1 activity was seen in the 20 Hz frequency range in all subjects. Interactions between the motor cortex and spinal motoneuron pool were investigated by separately studying the non-stationary phase and amplitude dynamics of M1 and EMG signals. Delays between M1 and EMG signals, computed from their phase difference, were found to be in agreement with conduction times from the primary motor cortex to the respective muscle. The time-dependent cortico-muscular phase synchronization was found to be correlated with the time course of both M1 and EMG signals. The findings demonstrate that the coupling between the primary motor cortex and motoneuron pool is at least partly due to phase synchronization of 20 Hz oscillations which varies over time. Furthermore, the consistent phase lag between M1 and EMG signals, compatible with conduction time between M1 and the respective muscle with the M1 activity preceding EMG activity, supports the conjecture that the motor cortex drives the motoneuron pool.
Figures



References
-
- Ahonen AI, Hämäläinen MS, Kajola MJ, Knuutila JET, Laine PP, Lounasmaa OV, Parkkonen LT, Simola JT, Tesche CD. 122-channel SQUID instrument for investigating the magnetic signals from the human brain. Physica Scripta. 1993;49:198–205.
-
- Auger F, Flandrin P, Goncalves P, Lemoine O. Time Frequency Toolbox. France: CNRS, Rice University, USA; 1999.
-
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Experimental Brain Research. 1999;128:109–117. - PubMed
-
- Brown P, Farmer SF, Halliday DM, Marsden J, Rosenberg JR. Coherent cortical and muscle discharge in cortical myoclonus. Brain. 1999;122:461–472. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

