Production of borreliacidal antibody to outer surface protein A in vitro and modulation by interleukin-4
- PMID: 10992445
- PMCID: PMC101497
- DOI: 10.1128/IAI.68.10.5496-5501.2000
Production of borreliacidal antibody to outer surface protein A in vitro and modulation by interleukin-4
Abstract
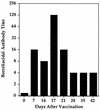
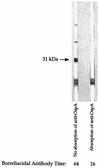
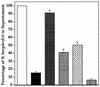
Borreliacidal antibody production is one of several parameters for establishing the effectiveness of Borrelia burgdorferi vaccines. The production of borreliacidal antibody was studied in vitro by culturing immune lymph node cells with macrophages and B. burgdorferi. We showed that borreliacidal antibody, directed primarily against outer surface protein A (OspA), was readily produced by lymph node cells obtained from C3H/HeJ mice vaccinated with formalin-inactivated B. burgdorferi in aluminum hydroxide, but not recombinant OspA. Anti-OspA borreliacidal antibody was detected in supernatants of cultures of lymph node cells obtained on day 7 after vaccination, peaked on day 17, and rapidly declined. The borreliacidal activity was attributable to immunoglobulin G1 (IgG1), IgG2a, and IgG2b antibodies. When lymph node cells were treated with interleukin-4 (IL-4), production of borreliacidal antibody was inhibited but was unaffected by treatment with anti-IL-4 antibodies. These results suggest that other cytokines, but not IL-4, are mainly responsible for production of the secondary borreliacidal antibody response.
Figures



Similar articles
-
Interleukin-6 promotes anti-OspA borreliacidal antibody production in vitro.Clin Vaccine Immunol. 2006 Jan;13(1):19-25. doi: 10.1128/CVI.13.1.19-25.2006. Clin Vaccine Immunol. 2006. PMID: 16425995 Free PMC article.
-
Gamma interferon inhibits production of Anti-OspA borreliacidal antibody in vitro.Clin Diagn Lab Immunol. 2002 Sep;9(5):1095-101. doi: 10.1128/cdli.9.5.1095-1101.2002. Clin Diagn Lab Immunol. 2002. PMID: 12204965 Free PMC article.
-
Interleukin-6 enhances production of anti-OspC immunoglobulin G2b borreliacidal antibody.Infect Immun. 2001 Jul;69(7):4268-75. doi: 10.1128/IAI.69.7.4268-4275.2001. Infect Immun. 2001. PMID: 11401963 Free PMC article.
-
A recombinant vaccine for Lyme disease.Behring Inst Mitt. 1994 Dec;(95):106-8. Behring Inst Mitt. 1994. PMID: 7755503 Review.
-
Vaccination as a modality to prevent Lyme disease. A status report.Infect Dis Clin North Am. 1999 Mar;13(1):135-48, vii. doi: 10.1016/s0891-5520(05)70047-7. Infect Dis Clin North Am. 1999. PMID: 10198796 Review.
Cited by
-
Neutralization of gamma interferon augments borreliacidal antibody production and severe destructive Lyme arthritis in C3H/HeJ mice.Clin Diagn Lab Immunol. 2004 Jan;11(1):35-41. doi: 10.1128/cdli.11.1.35-41.2004. Clin Diagn Lab Immunol. 2004. PMID: 14715542 Free PMC article.
-
New Zealand White Rabbits Effectively Clear Borrelia burgdorferi B31 despite the Bacterium's Functional vlsE Antigenic Variation System.Infect Immun. 2019 Jun 20;87(7):e00164-19. doi: 10.1128/IAI.00164-19. Print 2019 Jul. Infect Immun. 2019. PMID: 30988058 Free PMC article.
-
Hamster and murine models of severe destructive Lyme arthritis.Clin Dev Immunol. 2012;2012:504215. doi: 10.1155/2012/504215. Epub 2012 Feb 22. Clin Dev Immunol. 2012. PMID: 22461836 Free PMC article. Review.
-
The Development of a Rabies Virus-Vectored Vaccine against Borrelia burgdorferi, Targeting BBI39.Vaccines (Basel). 2024 Jan 12;12(1):78. doi: 10.3390/vaccines12010078. Vaccines (Basel). 2024. PMID: 38250891 Free PMC article.
-
Interleukin-6 promotes anti-OspA borreliacidal antibody production in vitro.Clin Vaccine Immunol. 2006 Jan;13(1):19-25. doi: 10.1128/CVI.13.1.19-25.2006. Clin Vaccine Immunol. 2006. PMID: 16425995 Free PMC article.
References
-
- Allione A, Bernabei P, Bosticardo M, Ariotti S, Forni G, Novelli F. Nitric oxide suppresses human T lymphocyte proliferation through IFN-γ-dependent and IFN-γ-independent induction of apoptosis. J Immunol. 1999;163:4182–4191. - PubMed
-
- Callister S M, Schell R F. Importance of protective borreliacidal antibodies in Lyme disease immunity and serodiagnosis. J Infect Dis. 1994;170:499–500. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

