Genetic vaccination against malaria infection by intradermal and epidermal injections of a plasmid containing the gene encoding the Plasmodium berghei circumsporozoite protein
- PMID: 10992502
- PMCID: PMC101554
- DOI: 10.1128/IAI.68.10.5914-5919.2000
Genetic vaccination against malaria infection by intradermal and epidermal injections of a plasmid containing the gene encoding the Plasmodium berghei circumsporozoite protein
Abstract
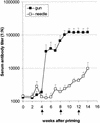
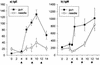
The circumsporozoite protein (CSP) from the surface of sporozoite stage Plasmodium sp. malaria parasites is among the most important of the malaria vaccine candidates. Gene gun injection of genetic vaccines encoding Plasmodium berghei CSP induces a significant protective effect against sporozoite challenge; however, intramuscular injection does not. In the present study we compared the immune responses and protective effects induced by P. berghei CSP genetic vaccines delivered intradermally with a needle or epidermally with a gene gun. Mice were immunized three times at 4-week intervals and challenged by a single infectious mosquito bite. Although 50 times more DNA was administered by needle than by gene gun, the latter method induced significantly greater protection against infection. Intradermal injection of the CSP genetic vaccine induced a strong Th1-type immune response characterized by a dominant CSP-specific immunoglobulin G2a (IgG2a) humoral response and high levels of gamma interferon produced by splenic T cells. Gene gun injection induced a predominantly Th2-type immune response characterized by a high IgG1/IgG2a ratio and significant IgE production. Neither method generated measurable cytotoxic T lymphocyte activity. The results indicate that a gene gun-mediated CS-specific Th2-type response may be best for protecting against malarial sporozoite infection when the route of parasite entry is via mosquito bite.
Figures

References
-
- Barry M A, Johnston S A. Biological features of genetic immunization. Vaccine. 1997;15:788–791. - PubMed
-
- Davis H L, Michel M L, Mancini M, Schleef M, Whalen R G. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine. 1994;12:1503–1509. - PubMed
-
- Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

