Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages
- PMID: 10993910
- PMCID: PMC2193285
- DOI: 10.1084/jem.192.6.789
Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages
Abstract
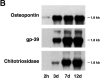
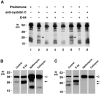
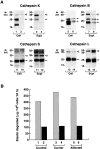
Human macrophages mediate the dissolution of elastic lamina by mobilizing tissue-destructive cysteine proteinases. While macrophage-mediated elastin degradation has been linked to the expression of cathepsins L and S, these cells also express cathepsin K, a new member of the cysteine proteinase family whose elastinolytic potential exceeds that of all known elastases. To determine the relative role of cathepsin K in elastinolysis, monocytes were differentiated under conditions in which they recapitulated a gene expression profile similar to that observed at sites of tissue damage in vivo. After a 12-d culture period, monocyte-derived macrophages (MDMs) expressed cathepsin K in tandem with cathepsins L and S. Though cysteine proteinases are acidophilic and normally confined to the lysosomal network, MDMs secreted cathepsin K extracellularly in concert with cathepsins L and S. Simultaneously, MDMs increased the expression of vacuolar-type H(+)-ATPase components, acidified the pericellular milieu, and maintained extracellular cathepsin K in an active form. MDMs from a cathepsin K-deficient individual, however, retained the ability to express, process, and secrete cathepsins L and S, and displayed normal elastin-degrading activity. Thus, matrix-destructive MDMs exteriorize a complex mix of proteolytic cysteine proteinases, but maintain full elastinolytic potential in the absence of cathepsin K by mobilizing cathepsins L and S.
Figures








Similar articles
-
Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages.Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3849-53. doi: 10.1073/pnas.92.9.3849. Proc Natl Acad Sci U S A. 1995. PMID: 7731994 Free PMC article.
-
Expression of cathepsin K messenger RNA in giant cells and their precursors in human osteoarthritic synovial tissues.Arthritis Rheum. 1999 Aug;42(8):1588-93. doi: 10.1002/1529-0131(199908)42:8<1588::AID-ANR4>3.0.CO;2-S. Arthritis Rheum. 1999. PMID: 10446855
-
Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages.FEBS Lett. 1988 Apr 11;231(1):229-31. doi: 10.1016/0014-5793(88)80737-3. FEBS Lett. 1988. PMID: 3360127
-
Elastin degradation by mononuclear phagocytes.Ann N Y Acad Sci. 1991;624:69-80. doi: 10.1111/j.1749-6632.1991.tb17007.x. Ann N Y Acad Sci. 1991. PMID: 2064250 Review.
-
Processing and activation of lysosomal proteinases.Biol Chem. 2002 Dec;383(12):1827-31. doi: 10.1515/BC.2002.206. Biol Chem. 2002. PMID: 12553719 Review.
Cited by
-
Macrophage cathepsin K promotes prostate tumor progression in bone.Oncogene. 2013 Mar 21;32(12):1580-93. doi: 10.1038/onc.2012.166. Epub 2012 May 21. Oncogene. 2013. PMID: 22614014 Free PMC article.
-
Pivotal role of cathepsin K in lung fibrosis.Am J Pathol. 2004 Jun;164(6):2203-16. doi: 10.1016/S0002-9440(10)63777-7. Am J Pathol. 2004. PMID: 15161653 Free PMC article.
-
Inflammation and immune response in COPD: where do we stand?Mediators Inflamm. 2013;2013:413735. doi: 10.1155/2013/413735. Epub 2013 Jul 15. Mediators Inflamm. 2013. PMID: 23956502 Free PMC article. Review.
-
Cathepsin K deficiency in mice induces structural and metabolic changes in the central nervous system that are associated with learning and memory deficits.BMC Neurosci. 2011 Jul 27;12:74. doi: 10.1186/1471-2202-12-74. BMC Neurosci. 2011. PMID: 21794126 Free PMC article.
-
Matrix metalloproteinases in emphysema.Matrix Biol. 2018 Nov;73:34-51. doi: 10.1016/j.matbio.2018.01.018. Epub 2018 Mar 23. Matrix Biol. 2018. PMID: 29406250 Free PMC article. Review.
References
-
- Carmeliet P., Moons L., Lijnen R., Baes M., Lemaitre V., Tipping P., Drew A., Eckhout Y., Shapiro S., Lupu F., Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 1997;17:439–444. - PubMed
-
- Hautamaki R.D., Kobayashi D.K., Senior R.M., Shapiro S.D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

