In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity
- PMID: 10993917
- PMCID: PMC2193284
- DOI: 10.1084/jem.192.6.871
In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity
Abstract
Multiple sclerosis (MS) is believed to be an autoimmune disease in which autoreactive T cells infiltrate the central nervous system (CNS). Animal models of MS have shown that CNS-specific T cells are present in the peripheral T cell repertoire of healthy mice and cause autoimmune disease only when they are activated by immunization. T cell entry into the CNS is thought to require some form of peripheral activation because the blood-brain barrier prohibits trafficking of this tissue by naive cells. We report here that naive T cells can traffic to the CNS without prior activation. Comparable numbers of T cells are found in the CNS of both healthy recombinase activating gene (Rag)(-/)- T cell receptor (TCR) transgenic mice and nontransgenic mice even when the transgenic TCR is specific for a CNS antigen. Transgenic T cells isolated from the CNS that are specific for non-CNS antigens are phenotypically naive and proliferate robustly to antigenic stimulation in vitro. Strikingly, transgenic T cells isolated from the CNS that are specific for myelin basic protein (MBP) are also primarily phenotypically naive but are unresponsive to antigenic stimulation in vitro. Mononuclear cells from the CNS of MBP TCR transgenic but not nontransgenic mice can suppress the response of peripheral MBP-specific T cells in vitro. These results indicate that naive MBP-specific T cells can traffic to the CNS but do not trigger autoimmunity because they undergo tolerance induction in situ.
Figures



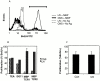

References
-
- Miller J.F., Heath W.R. Self-ignorance in the peripheral T-cell pool. Immunol. Rev. 1993;133:131–150. - PubMed
-
- Nossal G.J. Tolerance and ways to break it. Ann. NY. Acad. Sci. 1993;690:34–41. - PubMed
-
- Paterson P.Y. Experimental autoimmune (allergic) encephalomyelitisinduction, pathogenesis, and suppression. In: Mescher P.A., Mueller-Eberhard H.S., editors. Textbook of Immunopathology. Grune and Stratton; New York: 1976. pp. 179–213.
-
- Martin R., McFarland H.F. Immunology of multiple sclerosis and experimental allergic encephalomyelitis. In: Raine C.S., McFarland H.F., Tourtellotte W.W., editors. Multiple SclerosisClinical and Pathogenic Basis. Chapmand and Hall; London: 1997. pp. 221–242.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

