Thiol-mediated redox regulation of intestinal lamina propria T lymphocytes
- PMID: 10993921
- PMCID: PMC2193281
- DOI: 10.1084/jem.192.6.907
Thiol-mediated redox regulation of intestinal lamina propria T lymphocytes
Abstract
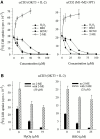
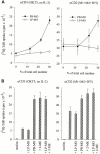
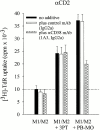
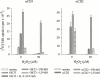
Intestinal lamina propria T lymphocytes (LP-Ts) have a markedly low proliferative potential both in vivo and in vitro. Here, we have identified that the capacity of antigen-presenting cells to release cysteine upon receptor-ligand interactions represents a critical parameter for proliferation of LP-Ts. The availability of cysteine is limiting for the intracellular production of glutathione, which in turn is essential for cell cycle progression. When cysteine is provided either directly or by addition of the reducing agent 2-mercaptoethanol to cystine-containing culture medium, proliferation of LP-T is fully restored. Importantly, coculture with peripheral blood monocytes that easily take up cystine, reduce cystine, and secrete cysteine also restores reactivity of LP-Ts to T cell receptor/CD3 stimulation. In marked contrast, lamina propria macrophages lack this capacity to elaborate cysteine, and thereby secure physiological unresponsiveness to antigen exposure in the intestinal microenvironment. The well-documented local recruitment of blood monocytes in inflammatory bowel disease (IBD) may thus represent an important parameter underlying hyperresponsiveness of T cells, an essential component of the pathogenesis of IBD.
Figures


Similar articles
-
A prominent role for mucosal cystine/cysteine metabolism in intestinal immunoregulation.Gastroenterology. 2008 Jan;134(1):179-91. doi: 10.1053/j.gastro.2007.11.001. Epub 2007 Nov 4. Gastroenterology. 2008. PMID: 18061179
-
Differential regulation of human T cell responsiveness by mucosal versus blood monocytes.Eur J Immunol. 1996 Apr;26(4):922-7. doi: 10.1002/eji.1830260430. Eur J Immunol. 1996. PMID: 8625989
-
Activation and signaling status of human lamina propria T lymphocytes.Gastroenterology. 1991 Dec;101(6):1529-36. doi: 10.1016/0016-5085(91)90388-2. Gastroenterology. 1991. PMID: 1955119
-
Molecular mechanisms securing "unresponsiveness" in lamina propria T lymphocytes.Ann N Y Acad Sci. 1996 Feb 13;778:174-84. doi: 10.1111/j.1749-6632.1996.tb21126.x. Ann N Y Acad Sci. 1996. PMID: 8610971 Review. No abstract available.
-
Epithelia: lymphocyte interactions in the gut.Immunol Rev. 2007 Feb;215:243-53. doi: 10.1111/j.1600-065X.2006.00484.x. Immunol Rev. 2007. PMID: 17291293 Free PMC article. Review.
Cited by
-
T cell metabolism drives immunity.J Exp Med. 2015 Aug 24;212(9):1345-60. doi: 10.1084/jem.20151159. Epub 2015 Aug 10. J Exp Med. 2015. PMID: 26261266 Free PMC article. Review.
-
The cystine/glutamate antiporter regulates dendritic cell differentiation and antigen presentation.J Immunol. 2010 Sep 15;185(6):3217-26. doi: 10.4049/jimmunol.1001199. Epub 2010 Aug 23. J Immunol. 2010. PMID: 20733204 Free PMC article.
-
Cofilin: a redox sensitive mediator of actin dynamics during T-cell activation and migration.Immunol Rev. 2013 Nov;256(1):30-47. doi: 10.1111/imr.12115. Immunol Rev. 2013. PMID: 24117811 Free PMC article. Review.
-
Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells.J Biol Chem. 2010 Dec 31;285(53):41525-32. doi: 10.1074/jbc.M110.189944. Epub 2010 Oct 30. J Biol Chem. 2010. PMID: 21037289 Free PMC article.
-
Immunoregulation in the gut: success and failures in human disease.Gut. 2002 May;50 Suppl 3(Suppl 3):III60-4. doi: 10.1136/gut.50.suppl_3.iii60. Gut. 2002. PMID: 11953335 Free PMC article. Review.
References
-
- Qiao L., Schürmann G., Betzler M., Meuer S.C. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology. 1991;101:1529–1536. - PubMed
-
- Targan S.R., Deem R.L., Liu M., Wang S., Nel A. Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J. Immunol. 1995;154:664–675. - PubMed
-
- De Maria R., Fais S., Silvestri M., Frati L., Pallone F., Santoni A., Testi R. Continuous in vivo activation and transient hyporesponsiveness to TcR/CD3 triggering of human gut lamina propria lymphocytes. Eur. J. Immunol. 1993;23:3104–3108. - PubMed
-
- Qiao L., Braunstein J., Golling M., Schürmann G., Autschbach F., Möller P., Meuer S.C. Differential regulation of human T cell responsiveness by mucosal versus blood monocytes. Eur. J. Immunol. 1996;26:922–927. - PubMed

