Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate
- PMID: 10995436
- PMCID: PMC2150699
- DOI: 10.1083/jcb.150.6.1299
Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate
Abstract
Neuronal Wiskott-Aldrich Syndrome protein (N-WASP) transmits signals from Cdc42 to the nucleation of actin filaments by Arp2/3 complex. Although full-length N-WASP is a weak activator of Arp2/3 complex, its activity can be enhanced by upstream regulators such as Cdc42 and PI(4,5)P(2). We dissected this activation reaction and found that the previously described physical interaction between the NH(2)-terminal domain and the COOH-terminal effector domain of N-WASP is a regulatory interaction because it can inhibit the actin nucleation activity of the effector domain by occluding the Arp2/3 binding site. This interaction between the NH(2)- and COOH termini must be intramolecular because in solution N-WASP is a monomer. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P(2)) influences the activity of N-WASP through a conserved basic sequence element located near the Cdc42 binding site rather than through the WASp homology domain 1. Like Cdc42, PI(4,5)P(2) reduces the affinity between the NH(2)- and COOH termini of the molecule. The use of a mutant N-WASP molecule lacking this basic stretch allowed us to delineate a signaling pathway in Xenopus extracts leading from PI(4, 5)P(2) to actin nucleation through Cdc42, N-WASP, and Arp2/3 complex. In this pathway, PI(4,5)P(2) serves two functions: first, as an activator of N-WASP; and second, as an indirect activator of Cdc42.
Figures


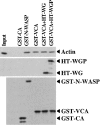
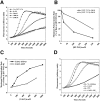
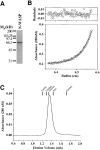



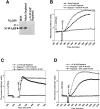

Comment in
-
How WASP regulates actin polymerization.J Cell Biol. 2000 Sep 18;150(6):F117-20. doi: 10.1083/jcb.150.6.f117. J Cell Biol. 2000. PMID: 10995455 Free PMC article. Review. No abstract available.
References
-
- Abdul-Manan N., Aghazadeh B., Liu G.A., Majumdar A., Ouerfelli O., Siminovitch K.A., Rosen M.K. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich Syndrome’ protein. Nature. 1999;399:379–383. - PubMed
-
- Aspenstrom P., Lindberg U., Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich Syndrome. Curr. Biol. 1996;6:70–75. - PubMed
-
- Banin S., Truong O., Katz D.R., Waterfield M.D., Brickell P.M., Gout I. Wiskott-Aldrich syndrome protein (WASp) is a binding partner for c-Src family protein-tyrosine kinases. Curr. Biol. 1996;6:981–988. - PubMed
-
- Bi E., Zigmond S.H. Actin polymerizationwhere the WASP stings. Curr. Biol. 1999;9:R160–R163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

