Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia
- PMID: 10995440
- PMCID: PMC2150709
- DOI: 10.1083/jcb.150.6.1349
Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia
Abstract
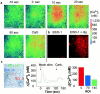
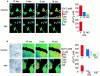
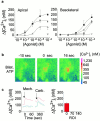
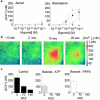
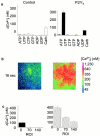
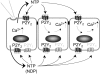
Airway epithelia are positioned at the interface between the body and the environment, and generate complex signaling responses to inhaled toxins and other stresses. Luminal mechanical stimulation of airway epithelial cells produces a propagating wave of elevated intracellular Ca(2+) that coordinates components of the integrated epithelial stress response. In polarized airway epithelia, this response has been attributed to IP(3) permeation through gap junctions. Using a combination of approaches, including enzymes that destroy extracellular nucleotides, purinergic receptor desensitization, and airway cells deficient in purinoceptors, we demonstrated that Ca(2+) waves induced by luminal mechanical stimulation in polarized airway epithelia were initiated by the release of the 5' nucleotides, ATP and UTP, across both apical and basolateral membranes. The nucleotides released into the extracellular compartment interacted with purinoceptors at both membranes to trigger Ca(2+) mobilization. Physiologically, apical membrane nucleotide-release coordinates airway mucociliary clearance responses (mucin and salt, water secretion, increased ciliary beat frequency), whereas basolateral release constitutes a paracrine mechanism by which mechanical stresses signal adjacent cells not only within the epithelium, but other cell types (nerves, inflammatory cells) in the submucosa. Nucleotide-release ipsilateral and contralateral to the surface stimulated constitutes a unique mechanism by which epithelia coordinate local and distant airway defense responses to mechanical stimuli.
Figures


References
-
- Abraham E.H., Okunieff P., Scala S., Vos P., Oosterveld M.J.S., Chen A.Y., Shrivastav B., Guidotti G. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science. 1997;275:1324–1325. - PubMed
-
- Boitano S., Dirksen E.R., Sanderson M.J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. - PubMed
-
- Boitano S., Sanderson M.J., Dirksen E.R. A role for Ca2+-conducting ion channels in mechanically-induced signal transduction of airway epithelial cells. J. Cell Sci. 1994;107:3037–3044. - PubMed
-
- Boitano S., Dirksen E.R., Evans W.H. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium. 1998;23:1–9. - PubMed
-
- Boyer J.L., Romero-Avila T., Schachter J.B., Harden T.K. Identification of competitive agonists of the P2Y1-receptor. Mol. Pharmacol. 1996;50:1323–1329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

