Single channel properties and regulated expression of Ca(2+) release-activated Ca(2+) (CRAC) channels in human T cells
- PMID: 10995447
- PMCID: PMC2150694
- DOI: 10.1083/jcb.150.6.1435
Single channel properties and regulated expression of Ca(2+) release-activated Ca(2+) (CRAC) channels in human T cells
Abstract
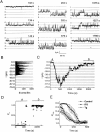
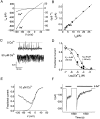
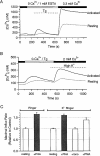
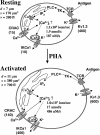
Although the crucial role of Ca(2+) influx in lymphocyte activation has been well documented, little is known about the properties or expression levels of Ca(2+) channels in normal human T lymphocytes. The use of Na(+) as the permeant ion in divalent-free solution permitted Ca(2+) release-activated Ca(2+) (CRAC) channel activation, kinetic properties, and functional expression levels to be investigated with single channel resolution in resting and phytohemagglutinin (PHA)-activated human T cells. Passive Ca(2+) store depletion resulted in the opening of 41-pS CRAC channels characterized by high open probabilities, voltage-dependent block by extracellular Ca(2+) in the micromolar range, selective Ca(2+) permeation in the millimolar range, and inactivation that depended upon intracellular Mg(2+) ions. The number of CRAC channels per cell increased greatly from approximately 15 in resting T cells to approximately 140 in activated T cells. Treatment with the phorbol ester PMA also increased CRAC channel expression to approximately 60 channels per cell, whereas the immunosuppressive drug cyclosporin A (1 microM) suppressed the PHA-induced increase in functional channel expression. Capacitative Ca(2+) influx induced by thapsigargin was also significantly enhanced in activated T cells. We conclude that a surprisingly low number of CRAC channels are sufficient to mediate Ca(2+) influx in human resting T cells, and that the expression of CRAC channels increases approximately 10-fold during activation, resulting in enhanced Ca(2+) signaling.
Figures



References
-
- Byrne J.A., Butler J.L., Cooper M.D. Differential activation requirements for virgin and memory T cells. J. Immunol. 1988;141:3249–3257. - PubMed
-
- Crabtree G.R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. - PubMed
-
- Crabtree G.R. Generic signals and specific outcomessignaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. - PubMed
-
- Crabtree G.R., Clipstone N.A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Ann. Rev. Biochem. 1994;63:1045–1083. - PubMed
-
- DeCoursey T.E., Chandy K.G., Gupta S., Cahalan M.D. Voltage-gated K+ channels in human T lymphocytesa role in mitogenesis? Nature. 1984;307:465–468. - PubMed

