Tor-mediated induction of autophagy via an Apg1 protein kinase complex
- PMID: 10995454
- PMCID: PMC2150712
- DOI: 10.1083/jcb.150.6.1507
Tor-mediated induction of autophagy via an Apg1 protein kinase complex
Abstract
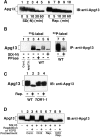
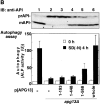
Autophagy is a membrane trafficking to vacuole/lysosome induced by nutrient starvation. In Saccharomyces cerevisiae, Tor protein, a phosphatidylinositol kinase-related kinase, is involved in the repression of autophagy induction by a largely unknown mechanism. Here, we show that the protein kinase activity of Apg1 is enhanced by starvation or rapamycin treatment. In addition, we have also found that Apg13, which binds to and activates Apg1, is hyperphosphorylated in a Tor-dependent manner, reducing its affinity to Apg1. This Apg1-Apg13 association is required for autophagy, but not for the cytoplasm-to-vacuole targeting (Cvt) pathway, another vesicular transport mechanism in which factors essential for autophagy (Apg proteins) are also employed under vegetative growth conditions. Finally, other Apg1-associating proteins, such as Apg17 and Cvt9, are shown to function specifically in autophagy or the Cvt pathway, respectively, suggesting that the Apg1 complex plays an important role in switching between two distinct vesicular transport systems in a nutrient-dependent manner.
Figures











References
-
- Beck T., Hall M.N. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

