Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis
- PMID: 10995788
- PMCID: PMC381392
- DOI: 10.1172/JCI9411
Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis
Abstract
Autodigestion of the pancreas by its own prematurely activated digestive proteases is thought to be an important event in the onset of acute pancreatitis. The mechanism responsible for the intrapancreatic activation of digestive zymogens is unknown, but a recent hypothesis predicts that a redistribution of lysosomal cathepsin B (CTSB) into a zymogen-containing subcellular compartment triggers this event. To test this hypothesis, we used CTSB-deficient mice in which the ctsb gene had been deleted by targeted disruption. After induction of experimental secretagogue-induced pancreatitis, the trypsin activity in the pancreas of ctsb(-/-) animals was more than 80% lower than in ctsb(+/+) animals. Pancreatic damage as indicated by serum activities of amylase and lipase, or by the extent of acinar tissue necrosis, was 50% lower in ctsb(-/-) animals. These experiments provide the first conclusive evidence to our knowledge that cathepsin B plays a role in intrapancreatic trypsinogen activation and the onset of acute pancreatitis.
Figures


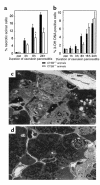

References
-
- Chiari H. Über die Selbstverdauung des menschlichen Pankreas. Zeitschrift für Heilkunde. 1896;17:69–96.
-
- Whitcomb DC. Early trypsinogen activation in acute pancreatitis. Gastroenterology. 1999;116:770–772. - PubMed
-
- Lerch MM, et al. Acute necrotising pancreatitis in the opossum: earliest morphologic changes involve acinar cells. Gastroenterology. 1992;103:205–213. - PubMed
-
- Whitcomb DC, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. - PubMed
-
- Gudgeon AM, et al. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990;335:4–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

