Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus
- PMID: 10995835
- PMCID: PMC6772844
- DOI: 10.1523/JNEUROSCI.20-18-06907.2000
Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus
Abstract
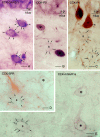
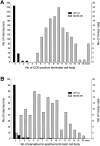
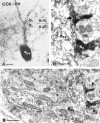
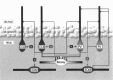
Perisomatic inhibitory innervation of all neuron types profoundly affects their firing characteristics and vulnerability. In this study we examined the postsynaptic targets of perisomatic inhibitory cells in the hilar region of the dentate gyrus where the proportion of potential target cells (excitatory mossy cells and inhibitory interneurons) is approximately equal. Both cholecystokinin (CCK)- and parvalbumin-immunoreactive basket cells formed multiple contacts on the somata and proximal dendrites of mossy cells. Unexpectedly, however, perisomatic inhibitory terminals arriving from these cell types largely ignored hilar GABAergic cell populations. Eighty-ninety percent of various GABAergic neurons including other CCK-containing basket cells received no input from CCK-positive terminals. Parvalbumin-containing cells sometimes innervated each other but avoided 75% of other GABAergic cells. Overall, a single mossy cell received 40 times more CCK-immunoreactive terminals and 15 times more parvalbumin-positive terminals onto its soma than the cell body of an average hilar GABAergic cell. In contrast to the pronounced target selectivity in the hilar region, CCK- and parvalbumin-positive neurons innervated each other via collaterals in stratum granulosum and moleculare. Our observations indicate that the inhibitory control in the hilar region is qualitatively different from other cortical areas at both the network level and the level of single neurons. The paucity of perisomatic innervation of hilar interneurons should have profound consequences on their action potential generation and on their ensemble behavior. These findings may help explain the unique physiological patterns observed in the hilus and the selective vulnerability of the hilar cell population in various pathophysiological conditions.
Figures





References
-
- Acsády L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996a;73:299–315. - PubMed
-
- Acsády L, Görcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996b;73:317–334. - PubMed
-
- Acsády L, Katona I, Gulyás AI, Shigemoto R, Freund TF. Immunostaining for substance P receptor labels GABAergic cells with distinct termination patterns in the hippocampus. J Comp Neurol. 1997;378:320–336. - PubMed
-
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus of the rat. J Comp Neurol. 1978;182:851–914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
