Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity
- PMID: 10995836
- PMCID: PMC6772815
- DOI: 10.1523/JNEUROSCI.20-18-06920.2000
Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity
Abstract
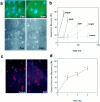
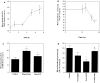
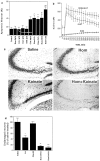
Elevated plasma levels of the sulfur-containing amino acid homocysteine increase the risk for atherosclerosis, stroke, and possibly Alzheimer's disease, but the underlying mechanisms are unknown. We now report that homocysteine induces apoptosis in rat hippocampal neurons. DNA strand breaks and associated activation of poly-ADP-ribose polymerase (PARP) and NAD depletion occur rapidly after exposure to homocysteine and precede mitochondrial dysfunction, oxidative stress, and caspase activation. The PARP inhibitor 3-aminobenzamide (3AB) protects neurons against homocysteine-induced NAD depletion, loss of mitochondrial transmembrane potential, and cell death, demonstrating a requirement for PARP activation and/or NAD depletion in homocysteine-induced apoptosis. Caspase inhibition accelerates the loss of mitochondrial potential and shifts the mode of cell death to necrosis; inhibition of PARP with 3AB attenuates this effect of caspase inhibition. Homocysteine markedly increases the vulnerability of hippocampal neurons to excitotoxic and oxidative injury in cell culture and in vivo, suggesting a mechanism by which homocysteine may contribute to the pathogenesis of neurodegenerative disorders.
Figures

References
-
- Adamec E, Vonsattel JP, Nixon RA. DNA strand breaks in Alzheimer's disease. Brain Res. 1999;849:67–77. - PubMed
-
- Andersson A, Brattstrom L, Israelsson B, Isaksson A, Hamfelt A, Hultberg B. Plasma homocysteine before and after methionine loading with regard to age, gender, and menopausal status. Eur J Clin Invest. 1992;22:79–87. - PubMed
-
- Bernofsky C, Swan M. An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem. 1973;53:452–460. - PubMed
-
- Blundell G, Jones BG, Rose FA, Tudball N. Homocysteine mediated endothelial cell toxicity and its amelioration. Atherosclerosis. 1996;122:163–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
