Determinants of skeletal muscle catabolism after severe burn
- PMID: 10998644
- PMCID: PMC1421178
- DOI: 10.1097/00000658-200010000-00001
Determinants of skeletal muscle catabolism after severe burn
Abstract
Objective: To determine which patient factors affect the degree of catabolism after severe burn.
Summary background data: Catabolism is associated with severe burn and leads to erosion of lean mass, impaired wound healing, and delayed rehabilitation.
Methods: From 1996 to 1999, 151 stable-isotope protein kinetic studies were performed in 102 pediatric and 21 adult subjects burned over 20-99. 5% of their total body surface area (TBSA). Patient demographics, burn characteristics, and hospital course variables were correlated with the net balance of skeletal muscle protein synthesis and breakdown across the leg. Data were analyzed sequentially and cumulatively through univariate and cross-sectional multiple regression.
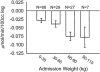
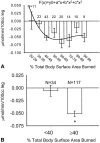
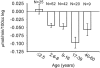
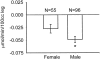
Results: Increasing age, weight, and delay in definitive surgical treatment predict increased catabolism (P < .05). Body surface area burned increased catabolism until 40% TBSA was reached; catabolism did not consistently increase thereafter. Resting energy expenditure and sepsis were also strong predictors of net protein catabolism. Among factors that did not significantly correlate were burn type, pneumonia, wound contamination, and time after burn. From these results, the authors also infer that gross muscle mass correlates independently with protein wasting after burn.
Conclusions: Heavier, more muscular subjects, and subjects whose definitive surgical treatment is delayed are at the greatest risk for excess catabolism after burn. Sepsis and excessive hypermetabolism are also associated with protein catabolism.
Figures


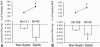
References
-
- Bessey PQ, Jiang Z, Johnson D, et al. Post-traumatic skeletal muscle proteolysis: The role of the hormonal environment. World J Surg 1989; 13: 465–470. - PubMed
-
- Ireton-Jones CS, Turner WW, Baxter CR. The effect of burn wound excision on measured energy expenditure and urinary nitrogen excretion. J Trauma 1987; 27 (2): 217–220. - PubMed
-
- Milner EA, Cioffi WG, Mason AD, et al. A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma 1994; 37 (2): 167–170. - PubMed
-
- Cuthbertson DP. Observations on disturbance of metabolism produced by injury to the limbs. Q J Med 1932; 25: 233.
-
- Cuthbertson DP, McGirr JL, Robertson JSM. The effect of fracture of bone on the metabolism of the rat. Q J Exp Physiol 1939; 29: 13.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

