Endovascular grafts and other image-guided catheter-based adjuncts to improve the treatment of ruptured aortoiliac aneurysms
- PMID: 10998645
- PMCID: PMC1421179
- DOI: 10.1097/00000658-200010000-00002
Endovascular grafts and other image-guided catheter-based adjuncts to improve the treatment of ruptured aortoiliac aneurysms
Abstract
Objective: To report a new management approach for the treatment of ruptured aortoiliac aneurysms.
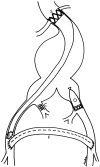
Methods: This approach includes hypotensive hemostasis, minimizing fluid resuscitation, and allowing the systolic blood pressure to fall to 50 mmHg. Under local anesthesia, a transbrachial guidewire was placed under fluoroscopic control in the supraceliac aorta. A 40-mm balloon catheter was inserted over this guidewire and inflated only if the blood pressure was less than 50 mmHg, before or after the induction of anesthesia. Fluoroscopic angiography was used to determine the suitability for endovascular graft repair. When possible, a prepared, "one-size-fits-most" endovascular aortounifemoral stented PTFE graft was used, combined with occlusion of the contralateral common iliac artery and femorofemoral bypass. If the patient's anatomy was unsuitable for endovascular graft repair, standard open repair was performed using proximal balloon control as needed.
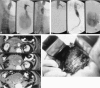
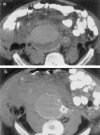
Results: Twenty-five patients with ruptured aortoiliac aneurysms (18 aortic, 7 iliac) were managed using this approach. Balloon inflation for proximal control was required in nine of the 25 patients. Twenty patients were treated with endovascular grafts. Five patients required open repair. The ruptured aneurysm was excluded in all 25 patients; 23 survived. Two deaths occurred in patients who received endovascular grafts with serious comorbidities. The surviving patients who received endovascular grafts had a median hospital stay of 6 days, and the preoperative symptoms resolved in all patients.
Conclusions: Hypotensive hemostasis is usually an effective means to provide time for balloon placement and often for endovascular graft insertion. With appropriate preparation and planning, many if not most patients with ruptured aneurysms can be treated by endovascular grafts. Proximal balloon control is not required often but may, when needed, be an invaluable adjunct to both endovascular graft and open repairs. The use of endovascular grafts and this approach using other image-guided catheter-based adjuncts appear to improve treatment outcomes for patients with ruptured aortoiliac aneurysms.
Figures



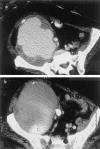
References
-
- Gerbode F. Ruptured abdominal aortic aneurysm: a surgical emergency. Surg Gynecol Obstet 1954; 98: 759–754.
-
- Ernst CB. Abdominal aortic aneurysms. N Engl J Med 1993; 328: 1167–1172. - PubMed
-
- Wakefield TW, Whitehouse WM, Wu SC. Abdominal aortic aneurysm rupture: statistical analysis of factors affecting outcome of surgical treatment. Surgery 1982; 91: 586–595. - PubMed
-
- Donaldson MC, Rosenberg JM, Bucknam CA. Factors affecting survival after ruptured abdominal aortic aneurysm. J Vasc Surg 1985; 2: 564–570. - PubMed
-
- Shackleton CR, Schechter MT, Bianco R, Hildebrand KD. Preoperative predictors of mortality risk in ruptured abdominal aortic aneurysm. J Vasc Surg 1987; 6: 583–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

