Complete repair of tetralogy of Fallot in the neonate: results in the modern era
- PMID: 10998649
- PMCID: PMC1421183
- DOI: 10.1097/00000658-200010000-00006
Complete repair of tetralogy of Fallot in the neonate: results in the modern era
Abstract
Objective: To review more than a decade of experience with complete repair of tetralogy of Fallot (TOF) in neonates at the University of Michigan; to assess early and late survival, perioperative complications, and the incidence of reoperation; and to analyze patient, procedural, and morphologic risk factors to determine their effects on outcome.
Summary background data: Palliation of TOF with systemic-to-pulmonary artery shunts has been the accepted standard for symptomatic neonates and infants. Complete repair has traditionally been reserved for infants older than 6 months of age because of the perception that younger and smaller infants face an unacceptably high surgical risk.
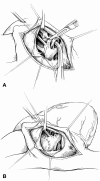
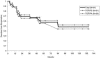
Results: A retrospective review from August 1988 to November 1999 consisted of 61 consecutive symptomatic neonates with TOF who underwent complete repair. Thirty-one patients had TOF with pulmonary stenosis, 24 had TOF with pulmonary atresia, and 6 had TOF with nonconfluent pulmonary arteries. The mean age at repair was 16 +/- 13 days, and the mean weight was 3.2 +/- 0.7 kg. Before surgery, 36 patients were receiving an infusion of prostaglandin, 26 were mechanically ventilated, and 11 required inotropic support. Right ventricular outflow tract obstruction was managed with a transannular patch in 49 patients and a right ventricle-to-pulmonary artery conduit in 12. Cardiopulmonary bypass time averaged 71 +/- 26 minutes. Hypothermic circulatory arrest was used in 52 patients (mean 38 +/- 12 minutes). After cardiopulmonary bypass, the average intraoperative right/left ventricular pressure ratio was 55% +/- 13%. There were no new clinically apparent neurologic sequelae after repair. The postoperative intensive care unit stay was 9.1 +/- 8 days, with 6.8 +/- 7 days of mechanical ventilation. There was one hospital death from postoperative necrotizing enterocolitis on postoperative day 71 and four late deaths, only one of which was cardiac-related. Actuarial survival was 93% at 5 years. Follow-up was available for all 60 hospital survivors and averaged 62 months (range 1-141 months). Twenty-two patients required a total of 24 reoperations at an average interval of 26 months after repair. Indications for reoperation included right ventricular outflow tract obstruction (19), branch pulmonary artery stenosis (11), severe pulmonary insufficiency (4), and residual ventricular septal defect (1). The 1-month, 1-year, and 5-year freedom from reoperation rates were 100%, 89%, and 58%, respectively.
Conclusions: Complete repair of TOF in the neonate is associated with excellent intermediate-term survival. Although the reoperation rate is significant, this is to be expected with the complex right ventricular outflow tract and pulmonary artery anatomy seen in symptomatic neonates and the need for conduit replacement in patients with TOF with pulmonary atresia.
Figures

References
-
- Fallot E. Contribution a l’anatomie pathologique de la maladie bleue (cyanose cardiaque). Marseille Med 1888; 25: 77ff. - PubMed
-
- Blalock A, Taussig HB. The surgical treatment of malformation of the heart in which there is pulmonary stenosis or pulmonary atresia. JAMA 1945; 128: 189. - PubMed
-
- Potts WJ, Smith S, Gibson S. Anastomosis of the aorta to a pulmonary artery. JAMA 1946; 132: 627. - PubMed
-
- Waterston DJ. Treatment of Fallot’s tetralogy in children under one year of age. Rozhl Chir 1962; 41: 181. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

