Evidence for a stable interaction of gp41 with Pr55(Gag) in immature human immunodeficiency virus type 1 particles
- PMID: 11000206
- PMCID: PMC112366
- DOI: 10.1128/jvi.74.20.9381-9387.2000
Evidence for a stable interaction of gp41 with Pr55(Gag) in immature human immunodeficiency virus type 1 particles
Abstract
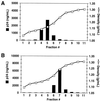
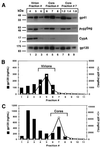
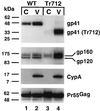
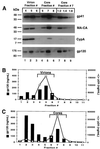
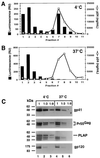
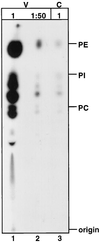
Assembly of infectious human immunodeficiency virus type 1 (HIV-1) virions requires incorporation of the viral envelope glycoproteins gp41 and gp120. Several lines of evidence have suggested that the cytoplasmic tail of the transmembrane glycoprotein, gp41, associates with Pr55(Gag) in infected cells to facilitate the incorporation of HIV-1 envelope proteins into budding virions. However, direct evidence for an interaction between gp41 and Pr55(Gag) in HIV-1 particles has not been reported. To determine whether gp41 is associated with Pr55(Gag) in HIV-1 particles, viral cores were isolated from immature HIV-1 virions by sedimentation through detergent. The cores contained a major fraction of the gp41 that was present on untreated virions. Association of gp41 with cores required the presence of the gp41 cytoplasmic tail. In HIV-1 particles containing a functional protease, a mutation that prevents cleavage of Pr55(Gag) at the matrix-capsid junction was sufficient for the detergent-resistant association of gp41 with the isolated cores. In addition to gp41, a major fraction of virion-associated gp120 was also detected on immature HIV-1 cores. Isolation of cores under conditions known to disrupt lipid rafts resulted in the removal of a raft-associated protein incorporated into virions but not the HIV-1 envelope proteins. These results provide biochemical evidence for a stable interaction between Pr55(Gag) and the cytoplasmic tail of gp41 in immature HIV-1 particles. Moreover, findings in this study suggest that the interaction of Pr55(Gag) with gp41 may regulate the function of the envelope proteins during HIV-1 maturation.
Figures
Similar articles
-
A mutation in the human immunodeficiency virus type 1 Gag protein destabilizes the interaction of the envelope protein subunits gp120 and gp41.J Virol. 2006 Mar;80(5):2405-17. doi: 10.1128/JVI.80.5.2405-2417.2006. J Virol. 2006. PMID: 16474147 Free PMC article.
-
Maturation-dependent human immunodeficiency virus type 1 particle fusion requires a carboxyl-terminal region of the gp41 cytoplasmic tail.J Virol. 2007 Sep;81(18):9999-10008. doi: 10.1128/JVI.00592-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609279 Free PMC article.
-
Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein.J Virol. 1996 Oct;70(10):6547-56. doi: 10.1128/JVI.70.10.6547-6556.1996. J Virol. 1996. PMID: 8794289 Free PMC article.
-
HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation.J Mol Biol. 2011 Jul 22;410(4):582-608. doi: 10.1016/j.jmb.2011.04.042. J Mol Biol. 2011. PMID: 21762802 Free PMC article. Review.
-
The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation.Viruses. 2020 May 16;12(5):548. doi: 10.3390/v12050548. Viruses. 2020. PMID: 32429351 Free PMC article. Review.
Cited by
-
The cytoplasmic tail domain of influenza B virus hemagglutinin is important for its incorporation into virions but is not essential for virus replication in cell culture in the presence of compensatory mutations.J Virol. 2012 Nov;86(21):11633-44. doi: 10.1128/JVI.01479-12. Epub 2012 Aug 15. J Virol. 2012. PMID: 22896616 Free PMC article.
-
Function of membrane rafts in viral lifecycles and host cellular response.Biochem Res Int. 2011;2011:245090. doi: 10.1155/2011/245090. Epub 2011 Dec 7. Biochem Res Int. 2011. PMID: 22191032 Free PMC article.
-
The frantic play of the concealed HIV envelope cytoplasmic tail.Retrovirology. 2013 May 24;10:54. doi: 10.1186/1742-4690-10-54. Retrovirology. 2013. PMID: 23705972 Free PMC article. Review.
-
HIV-1 Gag processing intermediates trans-dominantly interfere with HIV-1 infectivity.J Biol Chem. 2009 Oct 23;284(43):29692-703. doi: 10.1074/jbc.M109.027144. Epub 2009 Aug 7. J Biol Chem. 2009. PMID: 19666477 Free PMC article.
-
Kinetic studies of HIV-1 and HIV-2 envelope glycoprotein-mediated fusion.Retrovirology. 2006 Dec 4;3:90. doi: 10.1186/1742-4690-3-90. Retrovirology. 2006. PMID: 17144914 Free PMC article.
References
-
- Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
-
- Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

