Cytoplasmic domain signal sequences that mediate transport of varicella-zoster virus gB from the endoplasmic reticulum to the Golgi
- PMID: 11000211
- PMCID: PMC112371
- DOI: 10.1128/jvi.74.20.9421-9430.2000
Cytoplasmic domain signal sequences that mediate transport of varicella-zoster virus gB from the endoplasmic reticulum to the Golgi
Abstract
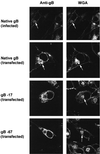
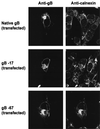
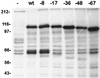
Normal herpesvirus assembly and egress depend on the correct intracellular localization of viral glycoproteins. While several post-Golgi transport motifs have been characterized within the cytoplasmic domains of various viral glycoproteins, few specific endoplasmic reticulum (ER)-to-Golgi transport signals have been described. We report the identification of two regions within the 125-amino-acid cytoplasmic domain of Varicella-Zoster virus gB that are required for its ER-to-Golgi transport. Native gB or gB containing deletions and specific point mutations in its cytoplasmic domain was expressed in mammalian cells. ER-to-Golgi transport of gB was assessed by indirect immunofluorescence and by the acquisition of Golgi-dependent posttranslational modifications. These studies revealed that the ER-to-Golgi transport of gB requires a nine-amino-acid region (YMTLVSAAE) within its cytoplasmic domain. Mutations of individual amino acids within this region markedly impaired the transport of gB from the ER to the Golgi, indicating that this domain functions by a sequence-dependent mechanism. Deletion of the C-terminal 17 amino acids of the gB cytoplasmic domain was also shown to impair the transport of gB from the ER to the Golgi. However, internal mutations within this region did not disrupt the transport of gB, indicating that its function during gB transport is not sequence dependent. Native gB is also transported to the nuclear membrane of transfected cells. gB lacking as many as 67 amino acids from the C terminus of its cytoplasmic domain continued to be transported to the nuclear membrane at apparently normal levels, indicating that the cytoplasmic domain of gB is not required for nuclear membrane localization.
Figures




Similar articles
-
Role of the varicella-zoster virus gB cytoplasmic domain in gB transport and viral egress.J Virol. 2002 Jan;76(2):591-9. doi: 10.1128/jvi.76.2.591-599.2002. J Virol. 2002. PMID: 11752150 Free PMC article.
-
Conserved cytoplasmic domain sequences mediate the ER export of VZV, HSV-1, and HCMV gB.Virology. 2004 Oct 10;328(1):131-41. doi: 10.1016/j.virol.2004.07.011. Virology. 2004. PMID: 15380364
-
VZV gB endocytosis and Golgi localization are mediated by YXXphi motifs in its cytoplasmic domain.Virology. 2001 Jun 20;285(1):42-9. doi: 10.1006/viro.2001.0930. Virology. 2001. PMID: 11414804
-
The why's of Y-based motifs in alphaherpesvirus envelope proteins.Virus Res. 2006 May;117(2):202-8. doi: 10.1016/j.virusres.2005.11.007. Epub 2006 Jan 18. Virus Res. 2006. PMID: 16417939 Review.
-
Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus.Curr Opin Cell Biol. 1994 Aug;6(4):517-21. doi: 10.1016/0955-0674(94)90070-1. Curr Opin Cell Biol. 1994. PMID: 7986527 Free PMC article. Review.
Cited by
-
Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion.J Virol. 2002 May;76(9):4390-400. doi: 10.1128/jvi.76.9.4390-4400.2002. J Virol. 2002. PMID: 11932406 Free PMC article.
-
Varicella-zoster virus: molecular controls of cell fusion-dependent pathogenesis.Biochem Soc Trans. 2020 Dec 18;48(6):2415-2435. doi: 10.1042/BST20190511. Biochem Soc Trans. 2020. PMID: 33259590 Free PMC article. Review.
-
Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression.J Virol. 2001 Oct;75(19):9483-92. doi: 10.1128/JVI.75.19.9483-9492.2001. J Virol. 2001. PMID: 11533210 Free PMC article.
-
Effects of mutations in the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B on intracellular transport and infectivity.J Virol. 2004 Feb;78(3):1540-51. doi: 10.1128/jvi.78.3.1540-1551.2004. J Virol. 2004. PMID: 14722308 Free PMC article.
-
Structure of Epstein-Barr virus tegument protein complex BBRF2-BSRF1 reveals its potential role in viral envelopment.Nat Commun. 2020 Oct 26;11(1):5405. doi: 10.1038/s41467-020-19259-x. Nat Commun. 2020. PMID: 33106493 Free PMC article.
References
-
- Campadelli G, Brandimarti R, Di Lazzaro C, Ward P L, Roizman B. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 1993;90:2798–2802. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

