A TVA-single-chain antibody fusion protein mediates specific targeting of a subgroup A avian leukosis virus vector to cells expressing a tumor-specific form of epidermal growth factor receptor
- PMID: 11000224
- PMCID: PMC112384
- DOI: 10.1128/jvi.74.20.9540-9545.2000
A TVA-single-chain antibody fusion protein mediates specific targeting of a subgroup A avian leukosis virus vector to cells expressing a tumor-specific form of epidermal growth factor receptor
Abstract
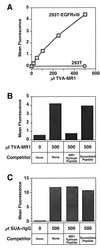
We have previously described an approach that employs retroviral receptor-ligand bridge proteins to target retroviral vectors to specific cell types. To determine whether targeted retroviral entry can also be achieved using a retroviral receptor-single-chain antibody bridge protein, the TVA-MR1 fusion protein was generated. TVA-MR1 is comprised of the extracellular domain of the TVA receptor for subgroup A avian leukosis viruses (ALV-A), fused to the MR1 single-chain antibody that binds specifically to EGFRvIII, a tumor-specific form of the epidermal growth factor receptor. We show that TVA-MR1 binds specifically to a murine version of EGFRvIII and promotes ALV-A entry selectively into cells that express this cell surface marker. These studies demonstrate that it is possible to target retroviral vectors to specific cell types through the use of a retroviral receptor-single-chain antibody fusion protein.
Figures


Similar articles
-
Targeting avian leukosis virus subgroup A vectors by using a TVA-VEGF bridge protein.J Virol. 2001 Feb;75(3):1571-5. doi: 10.1128/JVI.75.3.1571-1575.2001. J Virol. 2001. PMID: 11152532 Free PMC article.
-
Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein.Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):7063-8. doi: 10.1073/pnas.95.12.7063. Proc Natl Acad Sci U S A. 1998. PMID: 9618539 Free PMC article.
-
Targeting retroviral vector infection to cells that express heregulin receptors using a TVA-heregulin bridge protein.Virology. 2002 Jan 5;292(1):150-5. doi: 10.1006/viro.2001.1314. Virology. 2002. PMID: 11878917
-
Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion.Virology. 2006 Jan 5;344(1):25-9. doi: 10.1016/j.virol.2005.09.021. Virology. 2006. PMID: 16364732 Review.
-
Development of a flexible and specific gene delivery system for production of murine tumor models.Oncogene. 1999 Sep 20;18(38):5253-60. doi: 10.1038/sj.onc.1203087. Oncogene. 1999. PMID: 10498877 Review.
Cited by
-
Targeting avian leukosis virus subgroup A vectors by using a TVA-VEGF bridge protein.J Virol. 2001 Feb;75(3):1571-5. doi: 10.1128/JVI.75.3.1571-1575.2001. J Virol. 2001. PMID: 11152532 Free PMC article.
-
Similar regulation of cell surface human T-cell leukemia virus type 1 (HTLV-1) surface binding proteins in cells highly and poorly transduced by HTLV-1-pseudotyped virions.J Virol. 2002 Dec;76(24):12723-34. doi: 10.1128/jvi.76.24.12723-12734.2002. J Virol. 2002. PMID: 12438598 Free PMC article.
-
Retargeting of adenovirus vectors through genetic fusion of a single-chain or single-domain antibody to capsid protein IX.J Virol. 2010 Oct;84(19):10074-86. doi: 10.1128/JVI.02665-09. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631131 Free PMC article.
-
Oncolytic adenovirus retargeted to Delta-EGFR induces selective antiglioma activity.Cancer Gene Ther. 2009 Mar;16(3):256-65. doi: 10.1038/cgt.2008.75. Epub 2008 Oct 17. Cancer Gene Ther. 2009. PMID: 18927600 Free PMC article.
-
A fifteen-amino-acid TVB peptide serves as a minimal soluble receptor for subgroup B avian leukosis and sarcoma viruses.J Virol. 2002 Jun;76(11):5404-10. doi: 10.1128/jvi.76.11.5404-5410.2002. J Virol. 2002. PMID: 11991969 Free PMC article.
References
-
- Ager S, Nilson B H, Morling F J, Peng K W, Cosset F L, Russell S J. Retroviral display of antibody fragments; interdomain spacing strongly influences vector infectivity. Hum Gene Ther. 1996;7:2157–2164. - PubMed
-
- Bates P, Young J A T, Varmus H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. - PubMed
-
- Benedict C A, Tun R Y, Rubinstein D B, Guillaume T, Cannon P M, Anderson W F. Targeting retroviral vectors to CD34-expressing cells: binding to CD34 does not catalyze virus-cell fusion. Hum Gene Ther. 1999;10:545–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

