Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection
- PMID: 11000238
- PMCID: PMC112398
- DOI: 10.1128/jvi.74.20.9655-9667.2000
Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection
Abstract
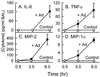
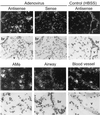
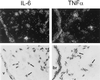
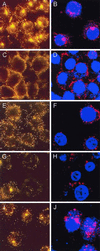
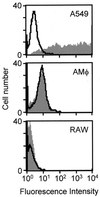
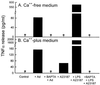
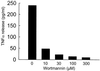
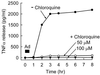
Adenovirus is a common respiratory pathogen which causes a broad range of distinct clinical syndromes and has recently received attention for its potential for in vivo gene delivery. Although adenovirus respiratory tract infection (ARTI) results in dose-dependent, local inflammation, the pathogenesis of this remains unclear. We hypothesized that alveolar macrophages (AMphi) rapidly internalize adenovirus following in vivo pulmonary administration and then initiate inflammatory signaling within the lung. To evaluate the role of AMphi in the induction of lung inflammation during ARTI in vivo, we directly assessed adenovirus uptake by murine AMphi and correlated uptake with the initiation of proinflammatory gene expression. Stimulation of cytokine (tumor necrosis factor alpha [TNF-alpha], interleukin-6 [IL-6], macrophage inflammatory protein-2 [MIP-2], and MIP-1alpha) expression in the lung was evaluated at the level of mRNA (by reverse transcription-PCR [RT-PCR]) and protein (by enzyme-linked immunosorbent assay) and by identification of cells expressing TNF-alpha and IL-6 mRNA in lung tissues (by in situ hybridization) and isolated lung lavage cells (by RT-PCR). Adenovirus, labeled with the fluorescent dye (Cy3), was rapidly and widely distributed on epithelial surfaces of airways and alveoli and was very rapidly ( approximately 1 min) localized within AMphi. At 30 min after infection AMphi but not airway epithelial or vascular endothelial cells expressed mRNA for TNF-alpha and IL-6, thus identifying AMphi as the cell source of initial cytokine signaling. IL-6, TNF-alpha, MIP-2, and MIP-1alpha levels progressively increased in bronchoalveolar lavage fluid after pulmonary adenovirus infection, and all were significantly elevated at 6 h (P < 0.05). To begin to define the molecular mechanism(s) by which adenovirus initiates the inflammatory signaling in macrophages, TNF-alpha expression from adenovirus-infected RAW264.7 macrophages was evaluated in vitro. TNF-alpha expression was readily detected in adenovirus-infected RAW cell supernatant with kinetics similar to AMphi during in vivo infection. Blockage of virus uptake at specific cellular sites, including internalization (by wortmannin), endosome acidification and/or lysis (by chloroquine) or by Ca(2+) chelation (by BAPTA) completely blocked TNF-alpha expression. In conclusion, results showed that during ARTI, (i) AMphi rapidly internalized adenovirus, (ii) expression of inflammatory mediators was initiated within AMphi and not airway epithelial or other cells, and (iii) the initiation of inflammatory signaling was linked to virion uptake by macrophages occurring at a point after vesicle acidification. These results have implications for our understanding of the role of the AMphi in the initiation of inflammation following adenovirus infection and adenovirus-mediated gene transfer to the lung.
Figures



Similar articles
-
Virulent and avirulent strains of equine arteritis virus induce different quantities of TNF-alpha and other proinflammatory cytokines in alveolar and blood-derived equine macrophages.Virology. 2003 Sep 30;314(2):662-70. doi: 10.1016/s0042-6822(03)00506-3. Virology. 2003. PMID: 14554093
-
Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses.Hum Gene Ther. 1998 Oct 10;9(15):2207-22. doi: 10.1089/hum.1998.9.15-2207. Hum Gene Ther. 1998. PMID: 9794205
-
SP-A enhances viral clearance and inhibits inflammation after pulmonary adenoviral infection.Am J Physiol. 1999 Sep;277(3):L580-8. doi: 10.1152/ajplung.1999.277.3.L580. Am J Physiol. 1999. PMID: 10484466
-
TNFalpha and MIP-2: role in particle-induced inflammation and regulation by oxidative stress.Toxicol Lett. 2000 Mar 15;112-113:177-83. doi: 10.1016/s0378-4274(99)00282-9. Toxicol Lett. 2000. PMID: 10720729 Review.
-
Adenovirus Entry: From Infection to Immunity.Annu Rev Virol. 2019 Sep 29;6(1):177-197. doi: 10.1146/annurev-virology-092818-015550. Epub 2019 Jul 5. Annu Rev Virol. 2019. PMID: 31283442 Review.
Cited by
-
Introduction of Two Prolines and Removal of the Polybasic Cleavage Site Lead to Higher Efficacy of a Recombinant Spike-Based SARS-CoV-2 Vaccine in the Mouse Model.mBio. 2021 Mar 2;12(2):e02648-20. doi: 10.1128/mBio.02648-20. mBio. 2021. PMID: 33653892 Free PMC article.
-
Advances in cell and gene-based therapies for cystic fibrosis lung disease.Mol Ther. 2012 Jun;20(6):1108-15. doi: 10.1038/mt.2012.32. Epub 2012 Feb 28. Mol Ther. 2012. PMID: 22371844 Free PMC article. Review.
-
Barriers to inhaled gene therapy of obstructive lung diseases: A review.J Control Release. 2016 Oct 28;240:465-488. doi: 10.1016/j.jconrel.2016.05.031. Epub 2016 May 16. J Control Release. 2016. PMID: 27196742 Free PMC article. Review.
-
Comparison of Transgenic and Adenovirus hACE2 Mouse Models for SARS-CoV-2 Infection.bioRxiv [Preprint]. 2020 Jul 6:2020.07.06.190066. doi: 10.1101/2020.07.06.190066. bioRxiv. 2020. Update in: Emerg Microbes Infect. 2020 Dec;9(1):2433-2445. doi: 10.1080/22221751.2020.1838955. PMID: 32676603 Free PMC article. Updated. Preprint.
-
Acanthamoeba castellanii is not be an adequate model to study human adenovirus interactions with macrophagic cells.PLoS One. 2017 Jun 7;12(6):e0178629. doi: 10.1371/journal.pone.0178629. eCollection 2017. PLoS One. 2017. PMID: 28591183 Free PMC article.
References
-
- Allen R D, Staley T A, Sidman C L. Differential cytokine expression in acute and chronic murine graft- versus-host-disease. Eur J Immunol. 1993;23:333–337. - PubMed
-
- Andiman W A, Miller G. Persistent infection with adenovirus types 5 and 6 in lymphoid cells from humans and woolly monkeys. J Infect Dis. 1982;145:83–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

