The vaccinia virus A9L gene encodes a membrane protein required for an early step in virion morphogenesis
- PMID: 11000242
- PMCID: PMC112402
- DOI: 10.1128/jvi.74.20.9701-9711.2000
The vaccinia virus A9L gene encodes a membrane protein required for an early step in virion morphogenesis
Abstract
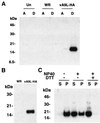
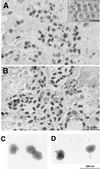
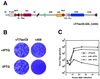
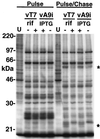
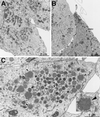
The A9L open reading frame of vaccinia virus was predicted to encode a membrane-associated protein. A transcriptional analysis of the A9L gene indicated that it was expressed at late times in vaccinia virus-infected cells. Late expression, as well as virion membrane association, was demonstrated by the construction and use of a recombinant vaccinia virus encoding an A9L protein with a C-terminal epitope tag. Immunoelectron microscopy revealed that the A9L protein was associated with both immature and mature virus particles and was oriented in the membrane with its C terminus exposed on the virion surface. To determine whether the A9L protein functions in viral assembly or infectivity, we made a conditional-lethal inducible recombinant vaccinia virus. In the absence of inducer, A9L expression and virus replication were undetectable. Under nonpermissive conditions, viral late protein synthesis occurred, but maturational proteolytic processing was inhibited, and there was an accumulation of membrane-coated electron-dense bodies, crescents, and immature virus particles, many of which appeared abnormal. We concluded that the product of the A9L gene is a viral membrane-associated protein and functions at an early stage in virion morphogenesis.
Figures




Similar articles
-
Vaccinia virus J1R protein: a viral membrane protein that is essential for virion morphogenesis.J Virol. 2002 Oct;76(19):9575-87. doi: 10.1128/jvi.76.19.9575-9587.2002. J Virol. 2002. PMID: 12208937 Free PMC article.
-
Vaccinia virus A17L gene product is essential for an early step in virion morphogenesis.J Virol. 1995 Aug;69(8):4640-8. doi: 10.1128/JVI.69.8.4640-4648.1995. J Virol. 1995. PMID: 7609028 Free PMC article.
-
Vaccinia virus A30L protein is required for association of viral membranes with dense viroplasm to form immature virions.J Virol. 2001 Jul;75(13):5752-61. doi: 10.1128/JVI.75.13.5752-5761.2001. J Virol. 2001. PMID: 11390577 Free PMC article.
-
Vaccinia virus A6L encodes a virion core protein required for formation of mature virion.J Virol. 2007 Feb;81(3):1433-43. doi: 10.1128/JVI.02206-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108027 Free PMC article.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
Cited by
-
Recombinant Sheep Pox Virus Proteins Elicit Neutralizing Antibodies.Viruses. 2016 Jun 7;8(6):159. doi: 10.3390/v8060159. Viruses. 2016. PMID: 27338444 Free PMC article.
-
Elements in the Development of a Production Process for Modified Vaccinia Virus Ankara.Microorganisms. 2013 Nov 1;1(1):100-121. doi: 10.3390/microorganisms1010100. Microorganisms. 2013. PMID: 27694766 Free PMC article. Review.
-
Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates.Virology. 2003 Feb 1;306(1):181-95. doi: 10.1016/s0042-6822(02)00038-7. Virology. 2003. PMID: 12620810 Free PMC article.
-
A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation.Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):12068-73. doi: 10.1073/pnas.210397997. Proc Natl Acad Sci U S A. 2000. PMID: 11035794 Free PMC article.
-
Vaccinia virus J1R protein: a viral membrane protein that is essential for virion morphogenesis.J Virol. 2002 Oct;76(19):9575-87. doi: 10.1128/jvi.76.19.9575-9587.2002. J Virol. 2002. PMID: 12208937 Free PMC article.
References
-
- Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. - PubMed
-
- Baldick C J, Moss B. Resistance of vaccinia virus to rifampicin conferred by a single nucleotide substitution near the predicted NH2 terminus of a gene encoding an Mr 62,000 polypeptide. Virology. 1987;156:138–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

