Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells
- PMID: 11000244
- PMCID: PMC112404
- DOI: 10.1128/jvi.74.20.9717-9726.2000
Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells
Abstract
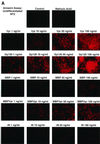
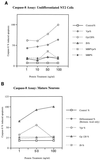
Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system (CNS) causes AIDS dementia complex (ADC) in certain infected individuals. Recent studies have suggested that patients with ADC have an increased incidence of neuronal apoptosis leading to neuronal dropout. Of note, a higher level of the HIV-1 accessory protein Vpr has been detected in the cerebrospinal fluid of AIDS patients with neurological disorders. Moreover, extracellular Vpr has been shown to form ion channels, leading to cell death of cultured rat hippocampal neurons. Based on these previous findings, we first investigated the apoptotic effects of the HIV-1 Vpr protein on the human neuronal precursor NT2 cell line at a range of concentrations. These studies demonstrated that apoptosis induced by both Vpr and the envelope glycoprotein, gp120, occurred in a dose-dependent manner compared to protein treatment with HIV-1 integrase, maltose binding protein (MBP), and MBP-Vpr in the undifferentiated NT2 cells. For mature, differentiated neurons, apoptosis was also induced in a dose-dependent manner by both Vpr and gp120 at concentrations ranging from 1 to 100 ng/ml, as demonstrated by both the terminal deoxynucleotidyltransferase (Tdt)-mediated dUTP-biotin nick end labeling and Annexin V assays for apoptotic cell death. In order to clarify the intracellular pathways and molecular mechanisms involved in Vpr- and gp120-induced apoptosis in the NT2 cell line and differentiated mature human neurons, we then examined the cellular lysates for caspase-8 activity in these studies. Vpr and gp120 treatments exhibited a potent increase in activation of caspase-8 in both mature neurons and undifferentiated NT2 cells. This suggests that Vpr may be exerting selective cytotoxicity in a neuronal precursor cell line and in mature human neurons through the activation of caspase-8. These data represent a characterization of Vpr-induced apoptosis in human neuronal cells, and suggest that extracellular Vpr, along with other lentiviral proteins, may increase neuronal apoptosis in the CNS. Also, identification of the intracellular activation of caspase-8 in Vpr-induced apoptosis of human neuronal cells may lead to therapeutic approaches which can be used to combat HIV-1-induced neuronal apoptosis in AIDS patients with ADC.
Figures




Similar articles
-
Critical implication of the (70-96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons.J Neurovirol. 2005 Dec;11(6):489-502. doi: 10.1080/13550280500384941. J Neurovirol. 2005. PMID: 16338743
-
Lentiviral expression of HIV-1 Vpr induces apoptosis in human neurons.J Neurovirol. 2002 Apr;8(2):86-99. doi: 10.1080/13550280290049552. J Neurovirol. 2002. PMID: 11935461
-
Ethanol potentiates HIV-1 gp120-induced apoptosis in human neurons via both the death receptor and NMDA receptor pathways.Virology. 2005 Mar 30;334(1):59-73. doi: 10.1016/j.virol.2005.01.014. Virology. 2005. PMID: 15749123
-
Effects of HIV-1 Vpr on neuroinvasion and neuropathogenesis.DNA Cell Biol. 2004 Apr;23(4):227-38. doi: 10.1089/104454904773819815. DNA Cell Biol. 2004. PMID: 15142380 Review.
-
Interactions of human immunodeficiency virus-1 proteins with neurons: possible role in the development of human immunodeficiency virus-1-associated dementia.Eur J Clin Invest. 2002 Aug;32(8):619-27. doi: 10.1046/j.1365-2362.2002.01029.x. Eur J Clin Invest. 2002. PMID: 12190962 Review.
Cited by
-
HIV-1 Vpu induces neurotoxicity by promoting Caspase 3-dependent cleavage of TDP-43.EMBO Rep. 2024 Oct;25(10):4337-4357. doi: 10.1038/s44319-024-00238-y. Epub 2024 Sep 6. EMBO Rep. 2024. PMID: 39242776 Free PMC article.
-
Human immunodeficiency virus type 1 Vpr-dependent cell cycle arrest through a mitogen-activated protein kinase signal transduction pathway.J Virol. 2005 Sep;79(17):11366-81. doi: 10.1128/JVI.79.17.11366-11381.2005. J Virol. 2005. PMID: 16103188 Free PMC article.
-
Machine Learning Prediction and Phyloanatomic Modeling of Viral Neuroadaptive Signatures in the Macaque Model of HIV-Mediated Neuropathology.Microbiol Spectr. 2023 Feb 27;11(2):e0308622. doi: 10.1128/spectrum.03086-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36847516 Free PMC article.
-
The effect of childhood trauma, ApoE genotype and HIV-1 viral protein R variants on change in cognitive performance.BMC Res Notes. 2019 Dec 27;12(1):828. doi: 10.1186/s13104-019-4869-9. BMC Res Notes. 2019. PMID: 31881924 Free PMC article.
-
Critical implication of the (70-96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons.J Neurovirol. 2005 Dec;11(6):489-502. doi: 10.1080/13550280500384941. J Neurovirol. 2005. PMID: 16338743
References
-
- Adie-Biassette H, Levy Y, Colombel M, Poron F, Natcher S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. - PubMed
-
- Bagasra O, Lavi E, Khalili K, Pestaner J P, Tawadros R, Pomerantz R J. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. - PubMed
-
- Boldin M P, Mett I L, Varfolomeev E E, Chumakov I, Shemer-Avni Y, Camonis J H, Wallach D. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995;270:387–391. - PubMed
-
- Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease in Fas/Apo-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

