Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than gag and is phosphorylated in infected cells
- PMID: 11000245
- PMCID: PMC112405
- DOI: 10.1128/jvi.74.20.9727-9731.2000
Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than gag and is phosphorylated in infected cells
Abstract
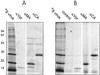
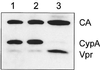
Viral protein R (Vpr) of human immunodeficiency virus type 1 (HIV-1) is a small accessory protein involved in the nuclear import of viral DNA and the growth arrest of host cells. Several studies have demonstrated that a significant amount of Vpr is incorporated into the virus particle via interaction with the p6 domain of Gag, and it is generally assumed that Vpr is packaged in equimolar ratio to Gag. We have quantitated the relative amount of Vpr in purified virions following [(35)S]cysteine labeling of infected MT-4 cells, as well as by quantitative immunoblotting and found that Vpr is present in a molar ratio of approximately 1:7 compared to capsid. Analysis of isolated core particles showed that Vpr is associated with the mature viral core, despite quantitative loss of p6 from core preparations. Metabolic labeling of infected cells with ortho[(32)P]phosphate revealed that a small fraction of Vpr is phosphorylated in virions and infected cells.
Figures



Similar articles
-
Incorporation of Vpr into human immunodeficiency virus type 1: role of conserved regions within the P6 domain of Pr55gag.J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Sep 1;10(1):1-7. J Acquir Immune Defic Syndr Hum Retrovirol. 1995. PMID: 7648278
-
Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6(gag).J Virol. 2000 Jul;74(13):6198-202. doi: 10.1128/jvi.74.13.6198-6202.2000. J Virol. 2000. PMID: 10846106 Free PMC article.
-
Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis.J Virol. 1993 Dec;67(12):7229-37. doi: 10.1128/JVI.67.12.7229-7237.1993. J Virol. 1993. PMID: 8230445 Free PMC article.
-
Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1.DNA Cell Biol. 2004 Apr;23(4):193-205. doi: 10.1089/104454904773819789. DNA Cell Biol. 2004. PMID: 15142377 Review.
-
Vpr and Its Cellular Interaction Partners: R We There Yet?Cells. 2019 Oct 24;8(11):1310. doi: 10.3390/cells8111310. Cells. 2019. PMID: 31652959 Free PMC article. Review.
Cited by
-
Proline 35 of human immunodeficiency virus type 1 (HIV-1) Vpr regulates the integrity of the N-terminal helix and the incorporation of Vpr into virus particles and supports the replication of R5-tropic HIV-1 in human lymphoid tissue ex vivo.J Virol. 2007 Sep;81(17):9572-6. doi: 10.1128/JVI.02803-06. Epub 2007 Jun 6. J Virol. 2007. PMID: 17553868 Free PMC article.
-
Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection.PLoS Pathog. 2008 May 9;4(5):e1000059. doi: 10.1371/journal.ppat.1000059. PLoS Pathog. 2008. PMID: 18464893 Free PMC article.
-
Virion-Associated Vpr Alleviates a Postintegration Block to HIV-1 Infection of Dendritic Cells.J Virol. 2017 Jun 9;91(13):e00051-17. doi: 10.1128/JVI.00051-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424288 Free PMC article.
-
De Novo Expressed Vpr Stimulates HIV-1 Replication in T Cells.Viruses. 2025 Jul 7;17(7):958. doi: 10.3390/v17070958. Viruses. 2025. PMID: 40733575 Free PMC article.
-
The Era of Gene Therapy: The Advancement of Lentiviral Vectors and Their Pseudotyping.Viruses. 2025 Jul 24;17(8):1036. doi: 10.3390/v17081036. Viruses. 2025. PMID: 40872751 Free PMC article. Review.
References
-
- Agostini I, Navarro J M, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. - PubMed
-
- Bachand F, Yao X J, Hrimech M, Rougeau N, Cohen E A. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 Gag precursor. J Biol Chem. 1999;274:9083–9091. - PubMed
-
- Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

