Critical role for the cysteines flanking the internal fusion peptide of avian sarcoma/leukosis virus envelope glycoprotein
- PMID: 11000247
- PMCID: PMC112407
- DOI: 10.1128/jvi.74.20.9738-9741.2000
Critical role for the cysteines flanking the internal fusion peptide of avian sarcoma/leukosis virus envelope glycoprotein
Abstract
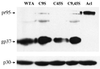
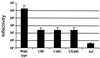
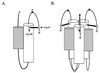
The transmembrane subunit (TM) of the envelope glycoprotein (Env) of the oncovirus avian sarcoma/leukosis virus (ASLV) contains an internal fusion peptide flanked by two cysteines (C9 and C45). These cysteines, as well as an analogous pair in the Ebola virus GP glycoprotein, are predicted to be joined by a disulfide bond. To examine the importance of these cysteines, we mutated C9 and C45 in the ASLV subtype A Env (EnvA), individually and together, to serine. All of the mutant EnvAs formed trimers that were composed of the proteolytically processed surface (SU) and TM subunits. All mutant EnvAs were incorporated into murine leukemia virus pseudotyped virions and bound receptor with wild-type affinity. Nonetheless, all mutant EnvAs were significantly impaired ( approximately 1,000-fold) in their ability to support infectivity. They were also significantly impaired in their ability to mediate cell-cell fusion. Our data are consistent with a model in which the internal fusion peptide of ASLV-A EnvA exists as a loop that is stabilized by a disulfide bond at its base and in which this stabilized loop serves an important function during virus-cell fusion. The fusion peptide of the Ebola virus GP glycoprotein may conform to a similar structure.
Figures


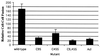
References
-
- Durell S R, Martin I, Ruysschaert J M, Shai Y, Blumenthal R. What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion. Mol Membr Biol. 1997;14:97–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

