Adenoviruses as vectors for delivering vaccines to mucosal surfaces
- PMID: 11000466
- PMCID: PMC7126179
- DOI: 10.1016/s0168-1656(00)00314-x
Adenoviruses as vectors for delivering vaccines to mucosal surfaces
Abstract
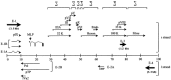
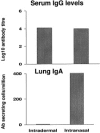
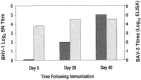
Immunization of mucosal surfaces has become an attractive route of vaccine delivery because of its ability to induce mucosal immunity. Although various methods of inducing mucosal immunity are being developed, our laboratory has focused on developing adenoviruses as replication-competent and replication-incompetent vectors. The present report will summarize our progress in sequencing the entire bovine adenovirus-3 genome and identifying regions which can be deleted and subsequently used as insertion sites for foreign genes in developing recombinant viral vaccines. Using these recombinant viruses, we demonstrated the 'proof-of-principle' in developing mucosal immunity and, more importantly, inducing protection against bovine herpes virus in a natural host-cattle. Finally, we demonstrated that immunity and protection occurred even in animals that had pre-existing antibodies to the vector.
Figures

References
-
- Arakawa, T., Chong, D.K., Langridge, W.H., 1998. Efficacy of a food plant-based oral cholera toxin B subunit vaccine [published erratum appears in Natl. Biotechnol. 16 (5), 478]. Natl. Biotechnol. 16, 292–297. - PubMed
-
- Babiuk L.A., L’Italien J., van Drunen Littel-van den Hurk S., Zamb T., Lawman M.J.P., Hughes G., Gifford G.A. Protection of cattle from bovine herpes virus type I (BHV-1) infection by immunization with individual viral glycoproteins. Virology. 1987;159:57–66. - PubMed
-
- Bowersock T.L., HogenEsch H., Suckow M., Guimond P., Martin S., Borie D., Torregrosa S., Park H., Park K. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine. 1999;17:1804–1811. - PubMed
-
- Brochier B., Pastoret P.P. Rabies eradication in Belgium by fox vaccination using vaccinia-rabies recombinant virus. Ondersterpoort J. Vet. Res. 1993;601:469–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

