Dynamic CT perfusion imaging of acute stroke
- PMID: 11003276
- PMCID: PMC7974057
Dynamic CT perfusion imaging of acute stroke
Abstract
Background and purpose: Because cerebral perfusion imaging for acute stroke is unavailable in most hospitals, we investigated the feasibility of a method of perfusion scanning that can be performed rapidly during standard cranial CT. Our aim was to identify the scanning parameters best suited to indicate tissue at risk and to measure a perfusion limit to predict infarction.
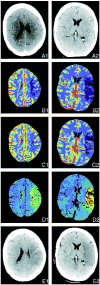
Methods: Seventy patients who had suffered stroke and had undergone cranial CT 0.5 to 12 hours (median, 3.75 hr) after the onset of symptoms participated in the study. While undergoing conventional CT, each patient received a bolus of iodinated contrast medium. Maps of time to peak (TTP), cerebral blood volume (CBV), and CBF were calculated from the resulting dynamically enhanced scans. These perfusion images were compared with follow-up CT scans or MR images showing the final infarctions.
Results: CBF maps predicted the extent of cerebral infarction with a sensitivity of 93% and a specificity of 98%. In contrast, CBV maps were less sensitive and TTP maps were less specific and also showed areas of collateral flow. Infarction occurred in all of the patients with CBF reduction of more than 70% and in half of the patients with CBF reduction of 40% to 70%.
Conclusion: Dynamic CT perfusion imaging safely detects tissue at risk in cases of acute stroke and is a feasible method for any clinic with a third-generation CT scanner.
Figures

References
-
- Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): second European Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245-1251 - PubMed
-
- The National Institute of Neurological Disorders and Stroke rTTPA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-1587 - PubMed
-
- Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study. JAMA 1995;274:1017-1025 - PubMed
-
- Wildermuth S, Knauth M, Brandt T, Winter R, Sartor K, Hacke W. Role of CT angiography in patient selection for thrombolytic therapy in acute hemispheric stroke. Stroke 1998;29:935-938 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
