Retroviral expression in embryonic stem cells and hematopoietic stem cells
- PMID: 11003639
- PMCID: PMC86295
- DOI: 10.1128/MCB.20.20.7419-7426.2000
Retroviral expression in embryonic stem cells and hematopoietic stem cells
Abstract
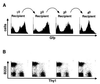
Achieving long-term retroviral expression in primary cells has been problematic. De novo DNA methylation of infecting proviruses has been proposed as a major cause of this transcriptional repression. Here we report the development of a mouse stem cell virus (MSCV) long terminal repeat-based retroviral vector that is expressed in both embryonic stem (ES) cells and hematopoietic stem (HS) cells. Infected HS cells and their differentiated descendants maintained long-term and stable retroviral expression after serial adoptive transfers. In addition, retrovirally infected ES cells showed detectable expression level of the green fluorescent protein (GFP). Moreover, GFP expression of integrated proviruses was maintained after in vitro differentiation of infected ES cells. Long-term passage of infected ES cells resulted in methylation-mediated silencing, while short-term expression was methylation independent. Tissues of transgenic animals, which we derived from ES cells carrying the MSCV-based provirus, did not express GFP. However, treatment with the demethylating agent 5-azadeoxycytidine reactivated the silent provirus, demonstrating that DNA methylation is involved in the maintenance of retroviral repression. Our results indicate that retroviral expression in ES cells is repressed by methylation-dependent as well as methylation-independent mechanisms.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
