Tumor suppressor p53 is required to modulate BRCA1 expression
- PMID: 11003642
- PMCID: PMC86298
- DOI: 10.1128/MCB.20.20.7450-7459.2000
Tumor suppressor p53 is required to modulate BRCA1 expression
Abstract
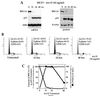
Individuals carrying mutations in BRCA1 or p53 genes are predisposed to a variety of cancers, and both tumor suppressor genes have been implicated in DNA damage response pathways. We have analyzed a possible functional link between p53 and BRCA1 genes. Here we show that BRCA1 expression levels are down-regulated in response to p53 induction in cells that undergo either growth arrest, senescence, or apoptosis. Physiological stimuli, such as exposure to DNA-damaging agents, also result in negative regulation of BRCA1 levels in a p53-dependent manner prior to causing cell cycle arrest. Nuclear run-on experiments and luciferase reporter assays demonstrate that the changes in BRCA1 expression are mainly due to transcriptional repression induced by p53. In conclusion, the data show that BRCA1 expression levels are controlled by the presence and activity of wild-type p53 and suggest the existence of an intracellular p53/BRCA1 pathway in the response of cells to stress conditions.
Figures




References
-
- Agarwal M L, Taylor W R, Chernov M V, Chernova O B, Stark G R. The p53 network. J Biol Chem. 1998;273:1–4. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Identification of newly transcribed RNA. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 4.10.1–4.10.7.
-
- Brawerman G, Belasco J G. Control of messenger RNA stability. San Diego, Calif: San Diego Academic Press; 1993.
-
- Castilla L H, Couch F J, Erdos M R, Hoskins K F, Calzone K, Garber J E, Boyd J, Lubin M B, Deshano M L, Brody L C, et al. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994;8:387–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
