Pore block versus intrinsic gating in the mechanism of inward rectification in strongly rectifying IRK1 channels
- PMID: 11004205
- PMCID: PMC2230623
- DOI: 10.1085/jgp.116.4.561
Pore block versus intrinsic gating in the mechanism of inward rectification in strongly rectifying IRK1 channels
Abstract
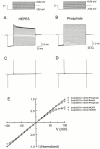
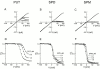
The IRK1 channel is inhibited by intracellular cations such as Mg(2+) and polyamines in a voltage-dependent manner, which renders its I-V curve strongly inwardly rectifying. However, even in excised patches exhaustively perfused with a commonly used artificial intracellular solution nominally free of Mg(2+) and polyamines, the macroscopic I-V curve of the channels displays modest rectification. This observation forms the basis of a hypothesis, alternative to the pore-blocking hypothesis, that inward rectification reflects the enhancement of intrinsic channel gating by intracellular cations. We find, however, that residual rectification is caused primarily by the commonly used pH buffer HEPES and/or some accompanying impurity. Therefore, inward rectification in the strong rectifier IRK1, as in the weak rectifier ROMK1, can be accounted for by voltage-dependent block of its ion conduction pore by intracellular cations.
Figures




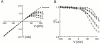
References
-
- Fakler B., Brandle U., Glowatzki E., Weidemann S., Zenner H.P., Ruppersburg J.P. Strong voltage-dependent inward-rectification of inward-rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. - PubMed
-
- Ficker E., Taglialatela M., Wible B.A., Henley C.M., Brown A.M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. - PubMed

