Cycloheximide- and puromycin-induced heat resistance: different effects on cytoplasmic and nuclear luciferases
- PMID: 11005376
- PMCID: PMC312884
- DOI: 10.1379/1466-1268(2000)005<0181:capihr>2.0.co;2
Cycloheximide- and puromycin-induced heat resistance: different effects on cytoplasmic and nuclear luciferases
Abstract
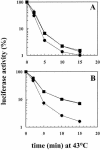
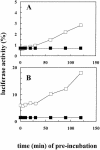
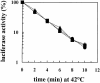
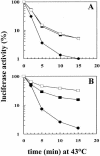
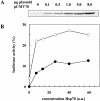
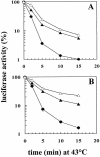
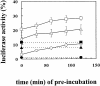
Inhibition of translation can result in cytoprotection against heat shock. The mechanism of this protection has remained elusive so far. Here, the thermoprotective effects of the translation inhibitor cycloheximide (CHX) and puromycin were investigated, using as reporter firefly luciferase localized either in the nucleus or in the cytoplasm. A short preincubation of O23 cells with either translation inhibitor was found to attenuate the heat inactivation of a luciferase directed into the cytoplasm, whereas the heat sensitivity of a nuclear-targeted luciferase remained unaffected. After a long-term CHX pretreatment, both luciferases were more heat resistant. Both the cytoplasmic and the nuclear luciferase are protected against heat-induced inactivation in thermotolerant cells and in cells overexpressing heat shock protein (Hsp)70. CHX incubations further attenuated cytoplasmic luciferase inactivation in thermotolerant and in Hsp70 overexpressing cells, even when Hsp70-mediated protection was saturated. It is concluded that protection by translation inhibition is unlikely due to an increase in the pool of free Hsps normally engaged in translation and released from the nascent polypeptide chains on the ribosomes. Rather, a decrease in nascent chains and thermolabile polypeptides may account for the heat resistance promoted by inhibitors of translation.
Figures
References
-
- Angelidis CE, Lazaridis I, Pagoulatos GN. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991;199:35–39. - PubMed
-
- Armour EP, Lee YJ, Corry PM, Borrelli MJ. Protection from heat-induced protein migration and DNA repair inhibition by cycloheximide. Biochem Biophys Res Commun. 1988;157:611–617. - PubMed
-
- Beckmann RP, Mizzen LA, Welch WJ. Interaction of Hsp70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. - PubMed
-
- Borrelli MJ, Stafford DM, Rausch CM, Lepock JR, Lee YJ, Corry PM. Reduction of levels of nuclear-associated protein in heated cells by cycloheximide, D2O, and thermotolerance. Radiat Res. 1992;131:204–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
