Aryl hydrocarbon (Ah) receptor levels are selectively modulated by hsp90-associated immunophilin homolog XAP2
- PMID: 11005382
- PMCID: PMC312890
- DOI: 10.1379/1466-1268(2000)005<0243:aharla>2.0.co;2
Aryl hydrocarbon (Ah) receptor levels are selectively modulated by hsp90-associated immunophilin homolog XAP2
Abstract
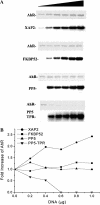
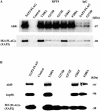
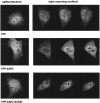
The aryl hydrocarbon receptor (AhR) is a ligand-inducible transcription factor that mediates biological responses to halogenated aromatic hydrocarbons. The unliganded AhR is a cytoplasmic, tetrameric complex consisting of the AhR ligand-binding subunit, a dimer of hsp90, and the hepatitis B virus X-associated protein 2 (XAP2). The role of XAP2 as a member of the AhR core complex is poorly understood. XAP2 shares significant homology with the immunophilins FKBP12 and FKBP52, including a highly conserved, C-terminal, tetratricopeptide repeat (TPR) domain. XAP2 forms a complex with hsp90 and the AhR but can also bind to both independently. This binding is mediated by the conserved TPR domain. Single-point mutations in this region are sufficient to disrupt the association of XAP2 with both the AhR and hsp90 in cells. Cotransfection of the AhR and XAP2 in COS-1 cells results in increased AhR levels compared with cells transfected with the AhR alone. In contrast, coexpression of the AhR with the TPR containing proteins FKBP52, protein phosphatase 5 (PP5), or XAP2 TPR-mutants deficient in binding to the AhR and hsp90 does not affect AhR levels and coexpression of the AhR with the TPR domain of PP5 results in AhR down-regulation. These results demonstrate that XAP2 is apparently unique among hsp90-binding proteins in its ability to enhance AhR levels. A yellow fluorescent protein (YFP)-XAP2-FLAG was constructed and biochemically characterized, and no loss of function was detected. YFP-XAP2-FLAG was transiently transfected into NIH 3T3 and was found to localize in both the nucleus and the cytoplasm when visualized by fluorescence microscopy. Treatment of Hepa-1 cells with the hsp90-binding benzoquinone ansamycin, geldanamycin, and the macrocyclic antifungal compound radicicol resulted in AhR but not XAP2 or FKBP52 turnover. Taken together, these results suggest that XAP2/hsp90 and FKBP52/hsp90 complexes are similar yet exhibit unique functional specificity.
Figures
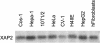




Similar articles
-
Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization.Biochemistry. 1999 Jul 13;38(28):8907-17. doi: 10.1021/bi982223w. Biochemistry. 1999. PMID: 10413464
-
A tetratricopeptide repeat half-site in the aryl hydrocarbon receptor is important for DNA binding and trans-activation potential.Mol Pharmacol. 2000 Dec;58(6):1517-24. doi: 10.1124/mol.58.6.1517. Mol Pharmacol. 2000. PMID: 11093792
-
Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity.Mol Cell Biol. 1998 Feb;18(2):978-88. doi: 10.1128/MCB.18.2.978. Mol Cell Biol. 1998. PMID: 9447995 Free PMC article.
-
The role of chaperone proteins in the aryl hydrocarbon receptor core complex.Chem Biol Interact. 2002 Sep 20;141(1-2):25-40. doi: 10.1016/s0009-2797(02)00064-9. Chem Biol Interact. 2002. PMID: 12213383 Review.
-
Functions of the Hsp90-binding FKBP immunophilins.Subcell Biochem. 2015;78:35-68. doi: 10.1007/978-3-319-11731-7_2. Subcell Biochem. 2015. PMID: 25487015 Review.
Cited by
-
Aryl hydrocarbon receptor: Its roles in physiology.Biochem Pharmacol. 2021 Mar;185:114428. doi: 10.1016/j.bcp.2021.114428. Epub 2021 Jan 28. Biochem Pharmacol. 2021. PMID: 33515530 Free PMC article. Review.
-
The aryl hydrocarbon receptor complex and the control of gene expression.Crit Rev Eukaryot Gene Expr. 2008;18(3):207-50. doi: 10.1615/critreveukargeneexpr.v18.i3.20. Crit Rev Eukaryot Gene Expr. 2008. PMID: 18540824 Free PMC article. Review.
-
Multi-chaperone function modulation and association with cytoskeletal proteins are key features of the function of AIP in the pituitary gland.Oncotarget. 2018 Jan 11;9(10):9177-9198. doi: 10.18632/oncotarget.24183. eCollection 2018 Feb 6. Oncotarget. 2018. PMID: 29507682 Free PMC article.
-
Novel functional association of serine palmitoyltransferase subunit 1-A peptide in sphingolipid metabolism with cytochrome P4501A1 transactivation and proliferative capacity of the human Glioma LN18 brain tumor cell line.Int J Environ Res Public Health. 2006 Sep;3(3):252-61. doi: 10.3390/ijerph2006030030. Int J Environ Res Public Health. 2006. PMID: 16968971 Free PMC article.
-
The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer.Cancer Metastasis Rev. 2008 Jun;27(2):169-78. doi: 10.1007/s10555-008-9125-z. Cancer Metastasis Rev. 2008. PMID: 18253812 Free PMC article. Review.
References
-
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. Analysis of FKBP51/FKBP52 chimeras and mutants for hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. - PubMed
-
- Bruner KL, Derfoul A, Robertson NM, Guerriero G, Fernandes-Alnemri T, Alnemri ES, Litwack G. The unliganded mineralocorticoid receptor is associated with heat shock proteins 70 and 90 and the immunophilin FKBP-52. Recept Signal Transduct. 1997;7:85–98. - PubMed
-
- Buchner J. Hsp90 & Co.—a holding for folding. TIBS. 1999;24:136–141. - PubMed
-
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. - PubMed
-
- Carrello A, Ingley E, Minchin RF, Tsai S, Ratajczak T. The common tetratricopeptide repeat acceptor site for steroid receptor-associated immunophilins and Hop is located in the dimerization domain of Hsp90. J Biol Chem. 1999;274:2682–2689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
