The Escherichia coli heat shock protein ClpB restores acquired thermotolerance to a cyanobacterial clpB deletion mutant
- PMID: 11005383
- PMCID: PMC312891
- DOI: 10.1379/1466-1268(2000)005<0255:techsp>2.0.co;2
The Escherichia coli heat shock protein ClpB restores acquired thermotolerance to a cyanobacterial clpB deletion mutant
Abstract
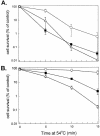
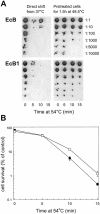
In both prokaryotes and eukaryotes, the heat shock protein ClpB functions as a molecular chaperone and plays a key role in resisting high temperature stress. ClpB is important for the development of thermotolerance in yeast and cyanobacteria but apparently not in Escherichia coli. We undertook a complementation study to investigate whether the ClpB protein from E coli (EcClpB) differs functionally from its cyanobacterial counterpart in the unicellular cyanobacterium Synechococcus sp. PCC 7942. The EcClpB protein is 56% identical to its ClpB1 homologue in Synechococcus. A plasmid construct was prepared containing the entire E coli clpB gene under the control of the Synechococcus clpB1 promoter. This construct was transformed into a Synechococcus clpB1 deletion strain (deltaclpB1) and integrated into a phenotypically neutral site of the chromosome. The full-length EcClpB protein (EcClpB-93) was induced in the transformed Synechococcus strain during heat shock as well as the smaller protein (EcClpB-79) that arises from a second translational start inside the single clpB message. Using cell survival measurements we show that the EcClpB protein can complement the Synechococcus deltaclpB1 mutant and restore its ability to develop thermotolerance. We also demonstrate that both EcClpB-93 and -79 appear to contribute to the degree of acquired thermotolerance restored to the Synechococcus complementation strains.
Figures



References
-
- Bustos SA, Golden SS. Light-regulated expression of the psbD gene family in Synechococcus sp. PCC 7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol Gen Genet. 1992;232:221–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
