Ribozyme rescue of photoreceptor cells in P23H transgenic rats: long-term survival and late-stage therapy
- PMID: 11005848
- PMCID: PMC17227
- DOI: 10.1073/pnas.210319397
Ribozyme rescue of photoreceptor cells in P23H transgenic rats: long-term survival and late-stage therapy
Abstract
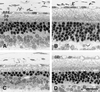
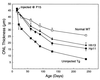
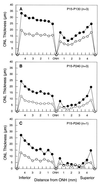
Ribozyme-directed cleavage of mutant mRNAs appears to be a potentially effective therapeutic measure for dominantly inherited diseases. We previously demonstrated that two ribozymes targeted to the P23H mutation in rhodopsin slow photoreceptor degeneration in transgenic rats for up to 3 months of age when injected before significant degeneration at postnatal day (P) 15. We now have explored whether ribozyme rescue persists at older ages, and whether ribozymes are effective when injected later in the degeneration after significant photoreceptor cell loss. Recombinant adeno-associated virus (rAAV) vectors incorporating a proximal bovine rod opsin promoter were used to transfer either hairpin or hammerhead ribozyme genes to photoreceptors. For the study of long-term survival, rAAV was administered by subretinal injection at P15, and the rats were allowed to live up to 8 months of age. For the study of late-stage gene transfer, rAAV was administered at P30 or P45, when 40-45% of the photoreceptors already had degenerated. Eyes were examined functionally by the electroretinogram and structurally by morphometric analysis. When injected at P15, expression of either ribozyme markedly slowed the rate of photoreceptor degeneration for at least 8 months and resulted in significantly greater electroretinogram amplitudes at least up to P180. When injected at P30 or P45, virtually the same number of photoreceptors survived at P130 as when injected at P15. Ribozyme rescue appears to be a potentially effective, long-term therapy for autosomal dominant retinal degeneration and is highly effective even when the gene transfer is done after significant photoreceptor cell loss.
Figures

References
-
- Humphries P, Farrar G J, Kenna P, McWilliam P. Clin Genet. 1990;38:1–13. - PubMed
-
- Berson E L. Invest Ophthalmol Visual Sci. 1993;34:1659–1676. - PubMed
-
- Gorin M B, Breitner J C S, De Jong P T V M, Hageman G S, Klaver C C W, Kuehn M H, Seddon J M. Mol Vis. 1999;5:29. - PubMed
-
- Zack D J, Dean M, Molday R S, Nathans J, Redmond T M, Stone E M, Swaroop A, Valle D, Weber B H. Mol Vis. 1999;5:30. - PubMed
-
- Wong F. Arch Ophthalmol. 1995;113:1245–1247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

