High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia
- PMID: 11005877
- PMCID: PMC27151
- DOI: 10.1073/pnas.97.20.11080
High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia
Abstract
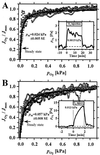
Mitochondria are confronted with low oxygen levels in the microenvironment within tissues; yet, isolated mitochondria are routinely studied under air-saturated conditions that are effectively hyperoxic, increase oxidative stress, and may impair mitochondrial function. Under hypoxia, on the other hand, respiration and ATP supply are restricted. Under these conditions of oxygen limitation, any compromise in the coupling of oxidative phosphorylation to oxygen consumption could accentuate ATP depletion, leading to metabolic failure. To address this issue, we have developed the approach of oxygen-injection microcalorimetry and ADP-injection respirometry for evaluating mitochondrial function at limiting oxygen supply. Whereas phosphorylation efficiency drops during ADP limitation at high oxygen levels, we show here that oxidative phosphorylation is more efficient at low oxygen than at air saturation, as indicated by higher ratios of ADP flux to total oxygen flux at identical submaximal rates of ATP synthesis. At low oxygen, the proton leak and uncoupled respiration are depressed, thus reducing maintenance energy expenditure. This indicates the importance of low intracellular oxygen levels in avoiding oxidative stress and protecting bioenergetic efficiency.
Figures



References
-
- Kasting J F. Science. 1993;259:920–926. - PubMed
-
- Martin W, Müller M. Nature (London) 1998;392:37–41. - PubMed
-
- Gray M W, Burger G, Lang B F. Science. 1999;283:1476–1481. - PubMed
-
- Hochachka P W, Lutz P L, Sick T, Rosenthal M, van den Thillart G, editors. Surviving Hypoxia: Mechanisms of Control and Adaptation. Boca Raton, FL: CRC; 1993.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

