Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma
- PMID: 11006226
- PMCID: PMC4318236
Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma
Abstract
Purpose: Gene targeted lumican-null mutants (lum(tm1sc)/lum(tm1sc)) have cloudy corneas with abnormally thick collagen fibrils. The purpose of the present study was to analyze the loss of transparency quantitatively and to define the associated corneal collagen fibril and stromal defects.
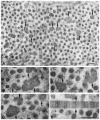
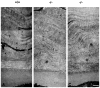
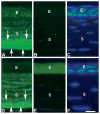
Methods: Backscattering of light, a function of corneal haze and opacification, was determined regionally using in vivo confocal microscopy in lumican-deficient and wild-type control mice. Fibril organization and structure were analyzed using transmission electron microscopy. Biochemical approaches were used to quantify glycosaminoglycan contents. Lumican distribution in the cornea was elucidated immunohistochemically. RESULTS; Compared with control stromas, lumican-deficient stromas displayed a threefold increase in backscattered light with maximal increase confined to the posterior stroma. Confocal microscopy through-focusing (CMTF) measurement profiles also indicated a 40% reduction in stromal thickness in the lumican-null mice. Transmission electron microscopy indicated significant collagen fibril abnormalities in the posterior stroma, with the anterior stroma remaining relatively unremarkable. The lumican-deficient posterior stroma displayed a pronounced increase in fibril diameter, large fibril aggregates, altered fibril packing, and poor lamellar organization. Immunostaining of wild-type corneas demonstrated high concentrations of lumican in the posterior stroma. Biochemical assessment of keratan sulfate (KS) content of whole eyes revealed a 25% reduction in KS content in the lumican-deficient mice.
Conclusions: The structural defects and maximum backscattering of light clearly localized to the posterior stroma of lumican-deficient mice. In normal mice, an enrichment of lumican was observed in the posterior stroma compared with that in the anterior stroma. Taken together, these observations indicate a key role for lumican in the posterior stroma in maintaining normal fibril architecture, most likely by regulating fibril assembly and maintaining optimal KS content required for transparency.
Figures



References
-
- McKusick VA. Mendelian Inheritance in Man. Baltimore, MD: The Johns Hopkins University Press; 1992.
-
- Alroy J, Haskins M, Birk D. Altered corneal stromal matrix organization is associated with mucopolysaccharidosis I, III and VI. Exp Eye Res. 1999;68:523–530. - PubMed
-
- Quantock AJ, Meek KM, Fullwood NJ, Zabel RW. Scheie’s syndrome: the architecture of corneal collagen and distribution of corneal proteoglycans. Can J Ophthalmol. 1993;28:266–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

