Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins
- PMID: 11007883
- PMCID: PMC6772789
- DOI: 10.1523/JNEUROSCI.20-19-07258.2000
Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins
Abstract
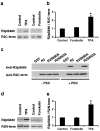
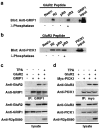
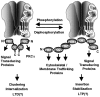
PSD-95, DLG, ZO-1 (PDZ) domain-mediated protein interactions have been shown to play important roles in the regulation of glutamate receptor function at excitatory synapses. Recent studies demonstrating the rapid regulation of AMPA receptor function during synaptic plasticity have suggested that AMPA receptor interaction with PDZ domain-containing proteins may be dynamically modulated. Here we show that PKC phosphorylation of the AMPA receptor GluR2 subunit differentially modulates its interaction with the PDZ domain-containing proteins GRIP1 and PICK1. The serine residue [serine-880 (Ser880)] in the GluR2 C-terminal sequence (IESVKI) critical for PDZ domain binding is a substrate of PKC and is phosphorylated in vivo. In vitro binding and coimmunoprecipitation studies show that phosphorylation of serine-880 within the GluR2 PDZ ligand significantly decreases GluR2 binding to GRIP1 but not to PICK1. Immunostaining of cultured hippocampal neurons demonstrates that the Ser880-phosphorylated GluR2 subunits are enriched and colocalized with PICK1 in the dendrites, with very little staining observed at excitatory synapses. Interestingly, PKC activation in neurons increases the Ser880 phosphorylation of GluR2 subunits and recruits PICK1 to excitatory synapses. Moreover, PKC stimulation in neurons results in rapid internalization of surface GluR2 subunits. These results suggest that GluR2 phosphorylation of serine-880 may be important in the regulation of the AMPA receptor internalization during synaptic plasticity.
Figures





References
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. - PubMed
-
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. - PubMed
-
- Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J Neurophysiol. 1998;80:2797–2800. - PubMed
-
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
